
Why SOPs are rarely followed, often cited, and in great need of follow-through.

Why SOPs are rarely followed, often cited, and in great need of follow-through.

A report commissioned by FDA evaluates the QbD paradigm.

US Pharmacopeia promotes horizontal standards and a product-class approach for quality attributes.

Rising imports, overseas production spur collaboration and realignment of enforcement activities.

Incorporating regulatory requirements into the product life cycle is crucial.
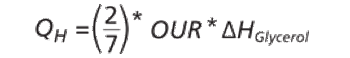
The authors describe challenges faced in transfer and scale-up of a fermentation process.

Follow-on versions of complex biologics require extensive expertise.

Industry struggles to curb drug abuse, diversion, and disruptions in supply to ensure access to quality products.

A hollow fiber matrix allows for efficient harvest of secreted proteins.

A rigorous cost-benefit assessment can help to chart a cost-effective path forward.

FDA, NIH, and industry seek new strategies to spur drug development and promote access to therapies.

Courts and Congress seek to reshape policies and programs affecting drug costs and access.

Executive management leadership is essential in the effective implementation of QbD.
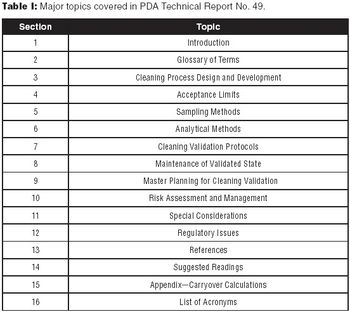
The authors encourage biotech manufacturers to consult PDA Technical Report No. 49 for a detailed perspective on current practices and issues in biotech cleaning validation.

As drug shortages make headlines, FDA tests the Sentinel safety system and its effect on healthcare.
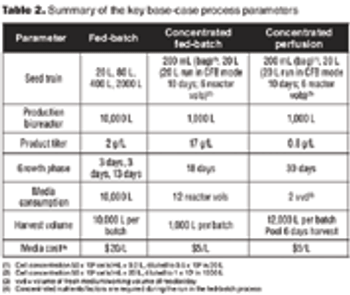
Comparing the economic feasibility of a typical glycosylated protein.

FDA prepares for top-level changes while promoting transparency and product safety

An approach to reduce batch time, increase productivity, and decrease costs.
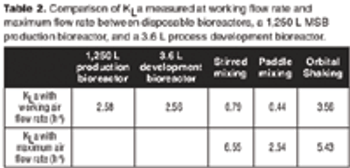
A case study to compare the performances of several types of mixing in disposable bags with stainless steel bioreactors.

Top priorities for manufacturers include user fees, new health initiatives, and regulatory compliance.

Changes on Capital Hill create uncertainty for healthcare reform, drug regulation, and biomedical research.

Best practices to strengthen supplier quality management.

Comparative effectiveness poses challenges for drug manufacturers.

A new strategy to streamline vaccine development and oversight.

Too many REMS cause headaches for doctors and the industry.