
Understanding the relationship between the process and CQAs.

Understanding the relationship between the process and CQAs.

FDA aims to regain public confidence in 2009.

QbD can help satisfy FDA and EMEA requirements.

MorphoSys AG (Munich, Germany) and Galapagos NV (Mechelen, Bengium) have launched a long-term co-development alliance aimed at discovering and developing antibody therapies based on novel modes of action in bone and joint disease, including rheumatoid arthritis, osteoporosis, and osteoarthritis.

To expand coverage amidst the economic crisis, Obama will be looking hard for ways to cut healthcare costs.
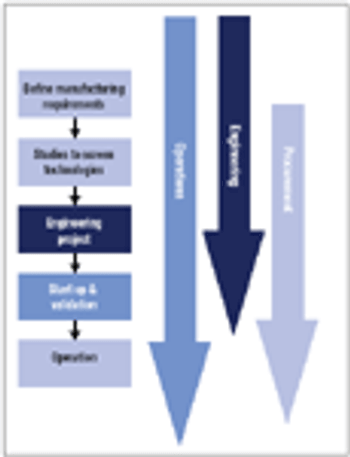
In new disposables projects, it is critical that engineering, procurement, and operations groups work together early on to manage supply chain risk.

No time for QbD? How to convince management to make it a priority.

The FDA's revised process validation guidance manages to explain the underlying concepts of Quality by Design without every using the phrase.
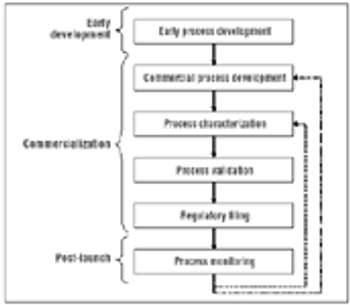
Using multivariate experiments to define acceptable ranges.

The FDA and other regulatory authorities are evaluating new regulations to ensure the safety and quality of nanomaterials in biomedical products.
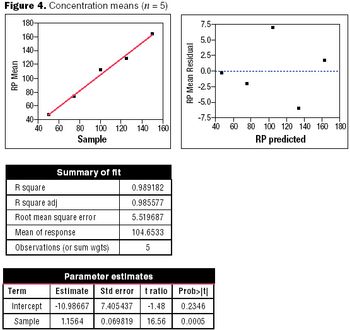
Well-designed experiments can reduce the risk of coming to an incorrect conclusion during a process characterization, assay validation, or process validation study.

The year 2007 witnessed the approval of fifteen biopharmaceuticals in the United States and European Union.

The FDA's QbD pilot program is supporting good manufacturing on a global basis.

In biomanufacturing today, there is increasing focus on improving process development. The goal is to accelerate development and reduce costs, without compromising the ability to scale up to a robust commercial process later on.

Every biotech company reaches a point in its development where it must decide what path it will take after it passes the start-up phase. This article discusses what the company must consider to decide what business model it will follow.

How to implement a risk-based approach to eliminating viruses.

The heparin debacle and other crises involving imported drugs and biologics has put pressure on the US FDA to step up its oversight of foreign drug manufacturing.
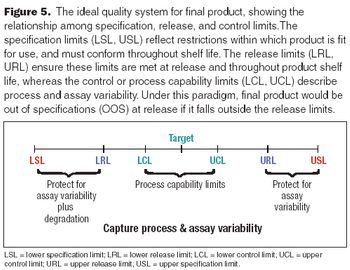
Set limits to provide incentives for process improvements.
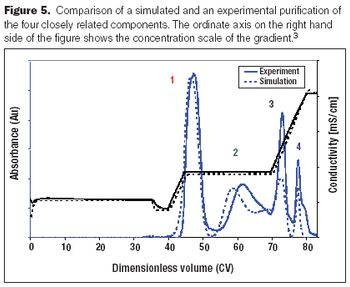
A review of some recent contributions in process chromatography.

The Sentinel System aims to generate more adverse event reporting by health professionals, to analyze health information more effectively, and to enhance FDA methods for communicating new safety information to providers and patients.
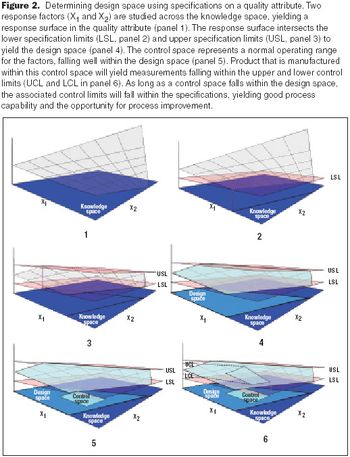
Quality by Design and Design Space can be used by companies to enhance process understanding, improve scientific rigor, and enhanced qualitative and quantative performance, as well as cost savings.

The US Food and Drugs Administration is boosting its efforts for orphan drugs development.
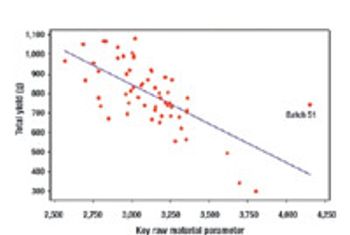
Resolve confusion about measurements.
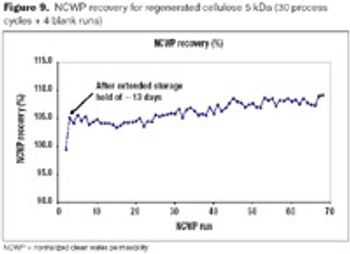
Best methods to maximize product yield and membrane lifetime to enhance a tangential flow filtration process.
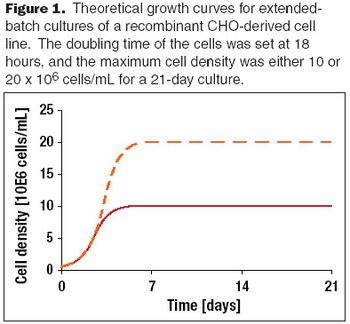
A discussion of past achievements and future expectations of recombinant protein production yields from mammalian cells.