
The comparative research approach may be preferable to price controls in the guise of government negotiations for the Medicare drug benefit, coverage denials, and limits on access to new technologies.


The comparative research approach may be preferable to price controls in the guise of government negotiations for the Medicare drug benefit, coverage denials, and limits on access to new technologies.

US Food and Drug Administration's Division of Biologic Oncology Products has approved two new biologics license application (BLA) supplements expanding the approval of Genentech's Herceptin (trastuzumab) for the treatment of breast cancer.

The FDA is under attack from all sides. Many influential members of Congress either don't trust the agency to monitor the industry appropriately, or have found it politically expedient to keep sounding alarms about inadequate oversight of food and drug safety and clinical research. The good news is that there seems to be a growing consensus that FDA needs a major infusion of cash to regain its stature as an effective science-based regulatory agency.
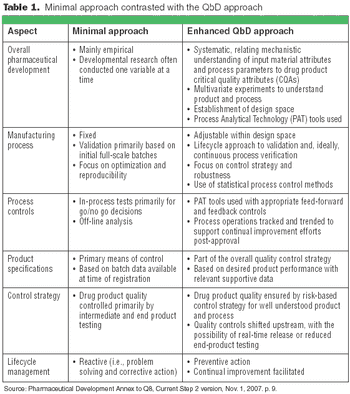
The principles of QbD can be applied to biotech development and manufacturing to help resolve many common issues. QbD scientifically provides a greater understanding of the complex relationships among product quality attributes, the manufacturing process, and clinical safety and efficacy by determining the various permutations of critical input variables that will keep the product within specification.
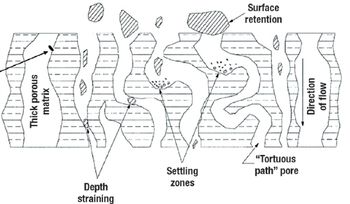
A comparison of primary harvest techniques.

Disposable technologies that mimic the conventional stainless-steel bioreactor will be most readily adopted

The heparin safety crisis puts a spotlight on manufacturing processes and regulatory oversight.
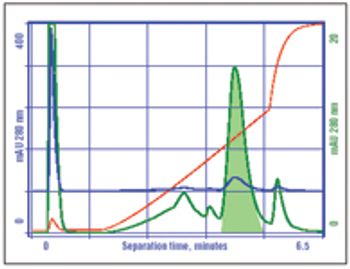
Manufacturing challenges surround the use of IgM monoclonal antibodies, but these can be overcome with current technology.
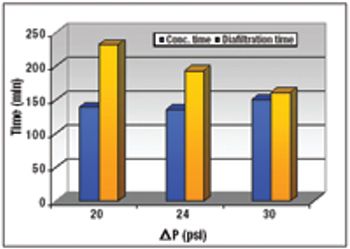
An alternative approach to traditional Protein A schemes is comparable in overall efficiency, product recovery, and quality.
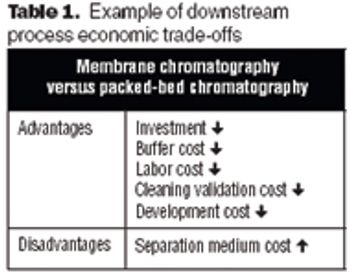
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.
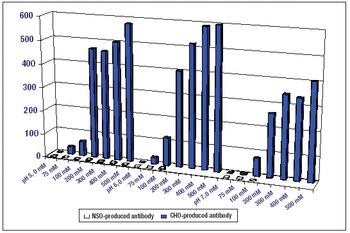
If certain engineering challenges can be addressed, precipitation may prove to be a valuable tool for antibody purification.

When platform processes are applied to fusion molecules, innovation and flexibility are needed.

More informed submissions may lead to regulatory flexibility for postapproval changes.
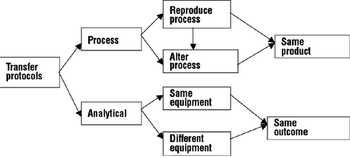
A comprehensive process and analytical transfer package can speed up your product's time to market and save costs.

On February 21, 2008, a congressional committee sent letters to Baxter Healthcare and the FDA seeking information related to the recent heparin recall.

The much needed modernization of the agency's IT systems and inspection capabilities will likely fall prey to budget shortfalls.

The use of disposables has changed significantly in the biopharmaceutical industry.

The new year begins on a note of optimism. A major breakthrough in stem cell research promises to open the door to new biomedical research opportunities. The drawn-out Congressional debate over user-fee reauthorization and drug safety regulation is over, and most parties seem satisfied with resulting compromises. The vaccine industry is experiencing a resurgence after years in the doldrums, with important new vaccines on the market and more under development. And unlike many previous years, the US Food and Drug Administration (FDA) had a confirmed commissioner for all of 2007 and relatively stable leadership.

In the process of developing breakthrough biopharmaceuticals with profound therapeutic promise, the many detailed requirements for a successful investigational new drug (IND) submission may seem petty, but they are not. With an IND, you are essentially moving from the cloistered world of the laboratory into a highly regulated industry where details not only matter, but are also greatly magnified by the overriding requirements of safety and efficacy. Treat those details with forethought and you will eventually succeed. Treat them as an afterthought and all of your pioneering science, state-of-the-art technology, and therapeutic ambition could come to nothing. At the very least, your progress to market could be delayed significantly. And if, like most young biopharmaceutical companies, you are on a short financial leash, such delays can be fatal for securing additional funding.

Design space concepts are key to a successful technology transfer.

The personalized medicine bandwagon is on a roll, offering a new model for calculating reimbursement of high-cost biotech therapies. Strategies for identifying patients who will respond to a certain therapy, as well as those most likely to suffer adverse events, promise to improve healthcare quality while eliminating waste and inappropriate spending. Interventions based on individual genetic characteristics may have limited sales, but support higher prices and less costly clinical research methods.
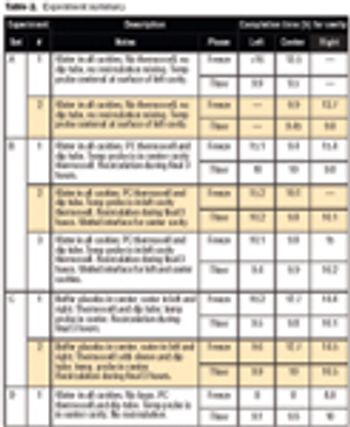
A solution for the problems of a "bag in a can" system would be a fully jacketed and insulated container, similar to a traditional freeze tank.

Congress postpones debate on follow-on biologics while adopting new policies likely to reshape drug development

Low-pressure process chromatography could not have developed without immense efforts to resolve scale-up issues in both column design and matrix stability.

It is commonly believed that technologies in the next 10–15 years will enable sequencing an individualized human genome for less than $1,000. With innovations like these, the twenty-first century will certainly belong to biotechnology. From an industrial standpoint, the discovery of therapeutic molecules and the development of cell lines and processes to produce these molecules will be of paramount importance. This article describes various approaches that have been prevalent in the industry or are likely to be used in the future for generating cell lines with desirable traits and developing high titer cell culture processes.