
The author presents best practices for extractables and leachables.

The author presents best practices for extractables and leachables.

Catalent Pharma Solutions has acquired a license to market Redwood Bioscience 's proprietary SMARTag precision protein-chemical engineering technology.

Astellas and Ambrx have entered into a collaboration to discover and develop novel antibody drug conjugates (ADCs) for an undisclosed number of targets in oncology. ADCs enable targeted delivery of drugs to the target tissue.

Have FDA initiatives improved manufacturing quality?

Latin America's diverse growing market seeks regulatory harmonization.

Opioid abuse generates calls for efforts to curb distribution.

Applications of ZFN technology in biopharmaceutical cell-line engineering.

EU authorities are stepping up their efforts to incorporate QbD principles.
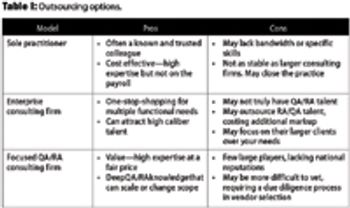
This article examines the options to best match needs and spending for quality and regulatory leadership.

The authors present solutions based on a review of current service offerings and their audit experience.

Vaccine development is benefiting from manufacturing advances.

Aggregate formation is influenced by multiple aspects of the bioproduction process but can be mitigated by good process design and control.

BioPharm International spoke with industry experts about the effect FDA's 2011 process validation guidance has had on industry.

USP's focus in 2013 involves standards relating to organic impurities, measurement of residual DNA and host-cell proteins in biotechnology products, and elemental impurities.

Discussions are underway as the pharmaceutical sector calls for greater consistency in the global monitoring of GMP compliance and quality testing of APIs and finished medicines.

Shortages spur efforts to overhaul manufacturing oversight.
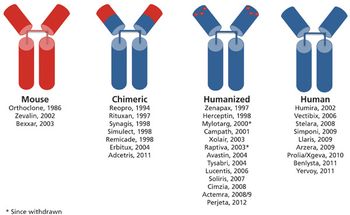
Innovative products and a range of indications drive the therapeutic antibody market.

Therapeutics targeting epigenetic mechanisms of disease will change the pharmaceutical marketplace.

Shortages spur efforts to overhaul manufacturing oversight.

The EU fine-tunes the Falsified Medicines Directive.

White House and Congress likely to struggle over funding for bio/pharmaceutical regulation.
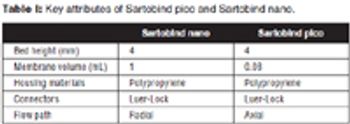
The authors describe the development of an ultra scale-down anion exchange membrane adsorber, and demonstrate scalability to larger-scale devices.

This month, Sharon Strause, an industry consultant, provides a look back at "Computer System Validation Part I: Testing and Verification of Applications Software" by Leonard J. Goren.
Insights on single-use systems implementation and exploitation in biopharmaceutical manufacturing and processing, based on a QbD approach.
Scaling up stem-cell cultures requires careful consideration of the bioreactor design.