Establishing the CQAs of a mAb product by evaluating impact and uncertainty during risk assessment.

Establishing the CQAs of a mAb product by evaluating impact and uncertainty during risk assessment.
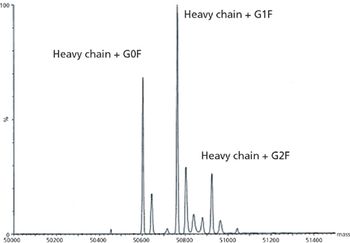
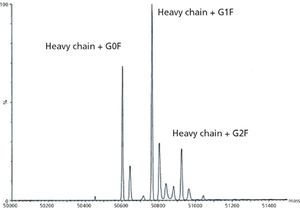
The author describes techniques that can be used to provide the analytical data required by ICH Q6B for characterization of monoclonal antibodies.
Published: August 2nd 2011 | Updated:
Published: July 1st 2014 | Updated: