Outsourcing analytics can be a cost-effective way for biopharma companies to adapt to new technologies and regulations.

Outsourcing analytics can be a cost-effective way for biopharma companies to adapt to new technologies and regulations.

Contract testing organizations can provide bio/pharma companies with a cost-effective way to adapt to new technologies and regulations.
Successful outsourcing relationships for early phase analytics in drug development are driven by partnership.
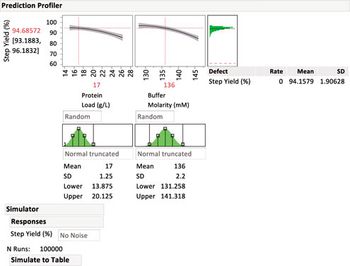
Statistical methods to identify critical process parameters and critical material attributes-and approaches to control them-are needed to protect drug product and drug substances.

The company aims to add the additional analytical services in the European Union throughout 2018.

Experts share insights on the various methods used for purity and impurity analysis of therapeutic proteins.

Corning will become the exclusive supplier of CytoSMART's new cell counter device that simplifies mammalian cell counting.

The critical quality attributes of biotherapeutics must be monitored to ensure product safety and efficacy.
The approaches for sample preparation of preclinical evaluation of safety and efficacy are addressed taking into consideration the shortcoming with the contemporary approaches.

Automated Control Concepts has launched Lab Owl bioreactor control system for labs using cell culture and fermentation applications.

The 2018 Short Course program at Pittcon, taking place Feb. 24–Mar. 1, will feature 23 new short courses geared towards laboratory professionals.

Conducting stability testing on APIs/finished drug product helps ensure shelf-life storage.
The authors present a robust and easy-to-implement chromatography column performance assessment method, called direct transition analysis (DTA).

SGS has introduced a Sanger sequencing service at its Glasgow, United Kingdom, laboratory to support genetic stability testing and perform identity testing on cell banks, plasmids, and viral seeds/vectors.

Biopharma majors are among the industry stakeholders who have commented and raised questions about FDA’s recently proposed draft guidance for analytical assessment of similarity in biosimilars.

A quality-by-design approach that implements PAT offers advantages in upstream cell-culture processing.

A roundtable Q&A with biopharma executives elucidates the challenges posed by single-use bioreactor bags in contributing to extractables and leachables in the biomanufacturing process.
Detecting viral contaminants in biologic-based medicines-and identifying their source-requires a holistic testing approach.

Process analytical testing for biopharmaceuticals requires enhanced methods due to complex bioprocesses.
A UHPLC SEC approach for protein aggregate analysis of mAbs is presented.

Industry experts weigh in on best practices, challenges, and mutual recognition of cleaning validation standards.

A new probe developed by researchers at the University of Melbourne, Australia, would allow NMR to be used without the use of microwaves, and with smaller machines.
While the measurement of the toxicity of leachables is not always a required parameter, the information collected during these studies could inform future bioprocessing runs.

How statistical methods and novel indices can be used to monitor and benchmark variability, to guide continuous improvement programs.

Operated by BioOutsource, Sartorius’ subsidiary, the Glasgow, UK-based service center will offer physicochemical properties and structural attributes testing and allow clients to perform structural and functional analyses in parallel.