
Despite limitations, mass spec is having an impact on biologic drug development and manufacturing.

Despite limitations, mass spec is having an impact on biologic drug development and manufacturing.

Extraction studies demonstrate approaches for evaluating single-use bio-pharmaceutical manufacturing materials.
Isothermal titration calorimetry and differential scanning calorimetry are valuable tools that can help accelerate drug development.

This report describes the first known attempt at quantifying the success of such processes in inducing nucleation on a 56–m2 freeze dryer operating at a load of 195,960 vials.
Understanding of endotoxin assays and a range of detection technologies are essential for effective testing.

To investigate the best culture conditions, the authors used response surface methodology via Box-Behnken design.
The development of mAb formulations poses challenges at the manufacturing, stability, analytical, and administration levels.

Interactions between biologic drug products and the components of prefilled syringes can cause protein aggregation, but there are alternative materials that can help mitigate this problem.

BioPharm International spoke with Trevor Marshall, director of enterprise systems integration at Zenith Technologies about automating processes in upstream processing.

The new guidelines will address bioanalytical method validation and biopharmaceutics classification system-based biowaivers.

The authors describe the qualification of an assay with applications for investigating functional comparability of an originator and biosimilar drug.
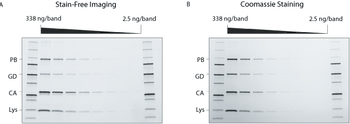
Innovations in electrophoresis and chromatography upstream of protein characterization can accelerate research.
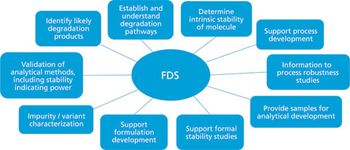
The author addresses critical issues to consider prior to performing forced degradation studies and provides best practice recommendations for these types of studies.

The assay, co-developed by Grifols and Hologic, will be used to test blood donations in the United States.

Investment at SGS’s Mississauga, Canada facility provides for analysis of molecular interactions in real time.
Data acquired from osmolality, glucose, and folic acid tests provides useful information for the specific identification of cell-culture media.
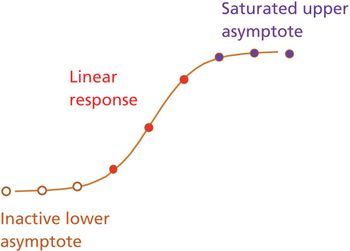
The author describes common components of a relative potency bioassay and provides a framework for assay development, calculation, and control.

A $1.1-million NSF grant awarded to Advanced Polymer Monitoring Technologies will be used for the development of a characterization technology for monitoring aggregation in biopharmaceuticals.

A new study concluded although some mAb products have heterogeneous variants, these charge variants are associated with similar potency and pharmacodynamic profiles as originator molecules.

Researchers describe a new method to compare the higher-order structure of a reference biologic with its proposed biosimilar product candidates.

Time and sensitivity are essential for analytical technologies in all phases of biopharma development.

The European Directorate for the Quality of Medicines & Healthcare announces the publication of a chemometric methods chapter in the European Pharmacopoeia.

The authors explore the use of precipitation using polyvinyl sulfonic acid and zinc chloride in place of capture chromatography to reduce the cost of goods in the insulin manufacturing process.
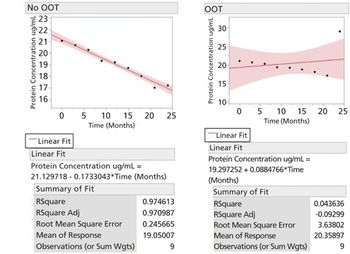
This article defines the concept, justification, and method of removal of out-of-trend points in stability modelling and shelf-life prediction.

The company’s new version of its glycan analysis tool will help investigators determine the relative abundance of individual N-glycan structures in small biopharmaceutical samples.