
Assessing biosafety using NGS-based tests requires a continuum of skills in molecular biology, biocomputing, virology, and quality systems.

Assessing biosafety using NGS-based tests requires a continuum of skills in molecular biology, biocomputing, virology, and quality systems.
Mass spectrometry and automation are growing in importance for protein characterization, but further improvements are still needed.
Dynamic light scattering presents a good analytical technique for testing protein stability.

The need for real-time monitoring and control has spurred the development of new analytical tools.

The companies intend to design and implement algorithms for the early stages of research for drug discovery and development of drug candidates to treat Alzheimer’s disease.

The acquisition strategically expands SPT Labtech’s offering in sample management for life sciences.

The new ultra-sensitive second-generation micro-chip technology is a key enabler in advanced single-cell proteomics workflow.

PathoQuest will apply its experience in viral safety testing and quality control of biologics for mitigating the risk of adventitious agent contamination in biopharmaceutical manufacturing.

Nexelis’ recent acquisition of GSK’s Marburg, Germany-based vaccines clinical bioanalytical laboratory expands its bioanalytical capabilities.

Vendors are finding ways to address the increasingly complex analytical challenges in the biopharmaceutical industry to further biotherapeutic development.

Clear understanding of what exactly the biomolecule entails is essential.

A novel PTA technology captures more than 95% of the genomes of single cells, providing more uniform, accurate, and reproducible single-cell analysis data.

The expansion of the companies’ partnership will integrate lead optimization efforts to advance cancer immune therapy drug discovery.

The partners have established PathoQuest, Inc., a US subsidiary, and will construct an NGS-based testing lab at Charles River’s Wayne, PA, site.
Early detection and more sensitive methods of detecting adventitious agents are becoming increasingly critical in bioprocessing.

Samsung Biologics has adopted Solentim’s cell seeding and cell metric platforms at its new R&D center in San Francisco, CA.

Mogrify’s new technology platform, EpiMOGRIFY, can predict cellular switches important for determining cell identity, cell maintenance, directed differentiation, and cell conversion.

With the presentation of research supporting the use of PTA technology, BioSkryb also launched its ResolveDNA platform for commercial use at ASHG 2020, held virtually on Oct. 27–30, 2020.

Analysts should understand how a monograph, together with the associated general notices and general chapters, relate to their responsibilities under good manufacturing practices.
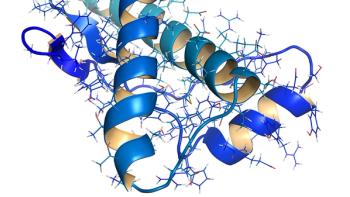
Glycoengineering is growing in importance as a technique to improve antibody therapeutic efficacy, safety, and product quality.

This article discusses standardized analytical method-transfer kits that have been developed to streamline method transfer and site certification at Eli Lilly. Use of these kits has been proven to improve overall efficiency and to reduce cost and time requirements for method transfer.

The company’s new CytoML Experiment Suite fully automates each stage of the flow cytometry data lifecycle, allowing for clearer data visualization and analysis.

The increased use of single-use bags in biologic manufacturing poses the risk of pinholes and other defects that cannot currently be tested for.

Current and newer biologic modalities pose increasingly complex challenges to the detection and characterization of protein aggregates.

Thermo Fisher Scientific is increasing access to cry-electron microscopy with the help of contract research organizations.