The authors present a review of the techniques commonly used for glycosylation analysis.

The authors present a review of the techniques commonly used for glycosylation analysis.

This article introduces the technology that powers automated HT–DLS and explores its practical applications in enhancing formulation stability investigations.
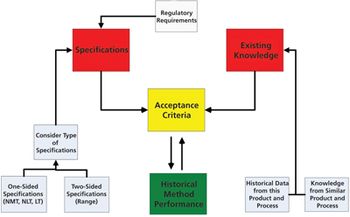
The authors present the results of a survey of biologics manufacturers to evaluate how these manufacturers transfer analytical methods.

Surface plasmon resonance is helping define bispecific antibodies, the next-generation of biopharma therapeutics.
Dynamic light scattering techniques can monitor viruses and virus-like particles in their native state.
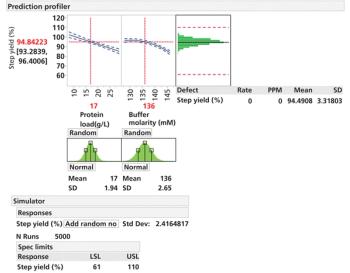
An approach to small-model generation and calibrating small-scale models to reliably predict performance at scale is presented.

Three case studies illustrate some analytical methods important for stability testing.

imagewerksBiophysical binding studies utilizing surface plasmon resonance, biolayer interferometry, isothermal titration calorimetry, or related techniques are ce

Sorendls/Getty ImagesTo maintain a state of control and comply with regulatory authorities, many pharmaceutical, biotech, and medical-device companies have adopted continued pro
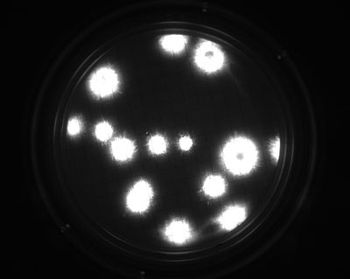
The rapid microbiological growth-based method represents an alternative for the quantification of contaminants in filterable products.

The authors explore the use of statistical experimental design and multivariate analysis to develop a drug substance formulation matrix.

The guidance provides recommendations for submitting analytical procedures and method validation data to FDA.

The collaboration will address the need for novel analytical approaches for the characterization of glycans.

The challenges and strategies of assessing and mitigating risk in biopharmaceutical manufacturing are discussed.

Advances in adventitious agent detection methodology are bringing benefits, but more work needs to be done.
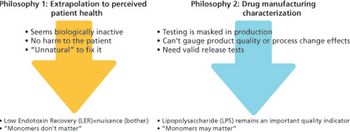
Low endotoxin recovery represents an opportunity to add value to the characterization of biologic drug products.
This article reviews the definition of HCPs, risks posed by HCPs, regulatory concerns, commonly accepted ELISA methods for HCP measurement and their limitations, and orthogonal methods available for HCP characterization.

Higher cell densities, greater demand for high-performance viral clearance, and desire for large-scale single-use technologies are driving development of filtration technologies.

The rapid testing of biologic raw materials can lead to greater efficiency.
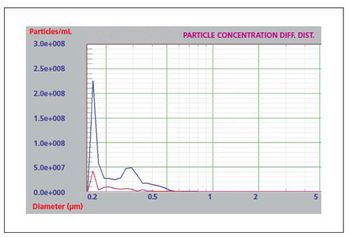
Liquid particle counters are ideal for protein aggregation studies.
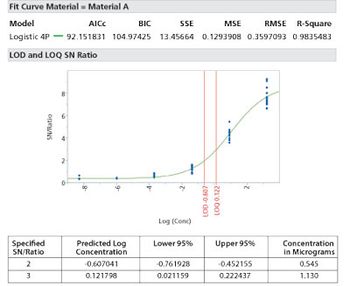
Care needs to be made to match the method of limit determination to the analytical method.
The thorough analysis of a therapeutic protein product’s propensity to aggregate may be a necessary step in the prevention of a cell-mediated immune response.

MedImmune will provide funds and access to monoclonal antibodies to seven postdoctoral associates for the creation of protein measurement and characterization tools.
As ADCs move through the drug-development process, different analytical methods are often required.