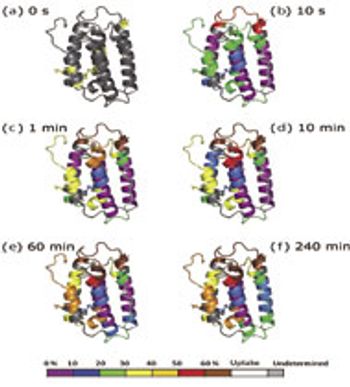
In HDX studies, data are produced across multiple time points, multiple species, and with replicates.

In HDX studies, data are produced across multiple time points, multiple species, and with replicates.
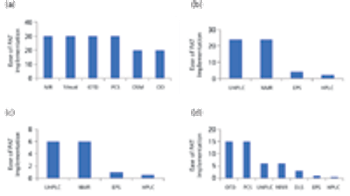
The authors review the various analytical methods that can enable use of PAT.
Current expectations in bioprocessing and a framework for using NMR to enhance a QbD approach.
Members from an ASQ working group provide analytical methods to enable PAT.

Unnecessary analytical testing can lead to unnecessary costs.
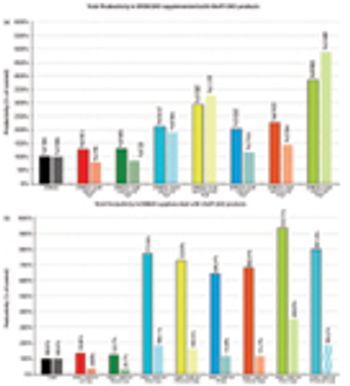
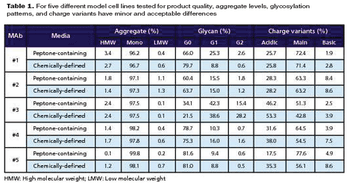
Cell-line specific complex media supplements combine chemically defined media additives into a single supplement.
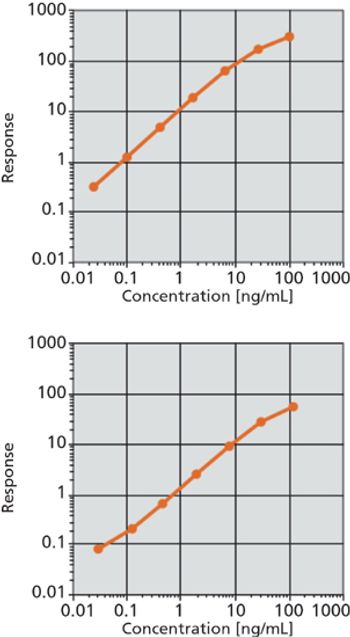
The authors discuss a new, rapid immunoassay for the detection of biomarkers.

Future sponsor-contract provider relationships will require more integration.

Rather than seeking a single indication for one large group, the orphan-drug approach segments the market for a drug more minutely.
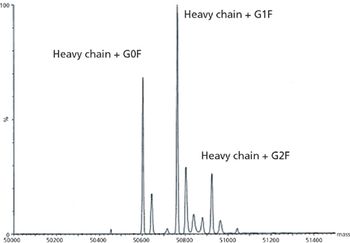
The author describes techniques that can be used to provide the analytical data required by ICH Q6B for characterization of monoclonal antibodies.
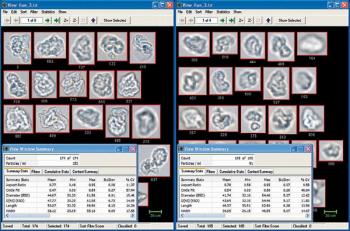
Overcoming limitations of volumetric techniques and detecting transparent particles.
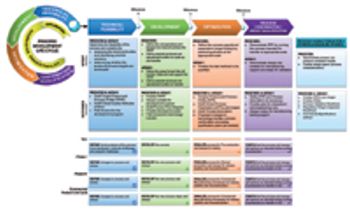
Achieving multiproduct development within shortened timelines.
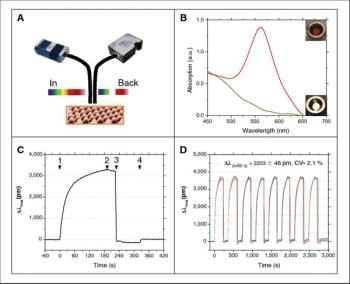
Use it label-free, or add labels to detect contaminants in solution.
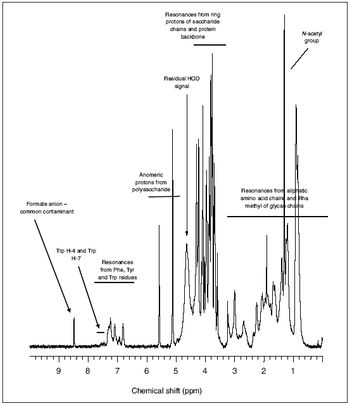
Simple methods can characterize polysaccharide vaccines and recombinant cytokines at high resolution.
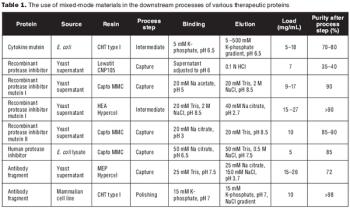
Salt-tolerant adsorption and unique selectivity are the major advantages of mixed-mode materials over single-mode resins.

Membrane chromatography ensures purity at high flow rates.
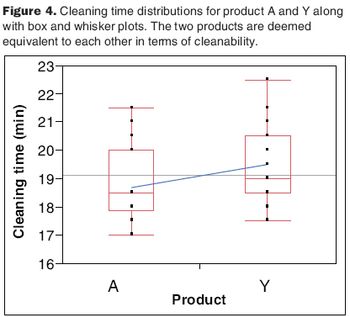
The two-one-sided t-test compares the equivalency of two data sets.
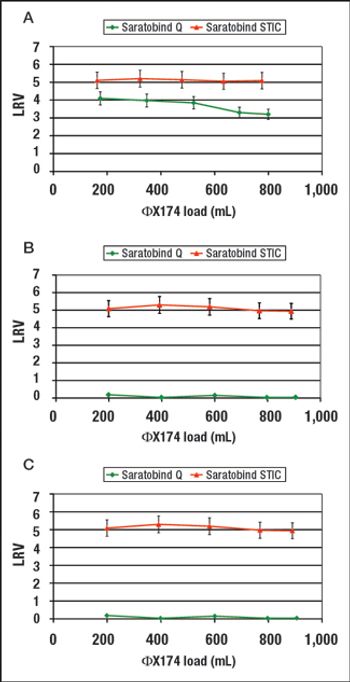
STIC allows polishing to be carried out without an interstitial dilution step, which reduces process time and avoids additional buffer preparation and hold steps.
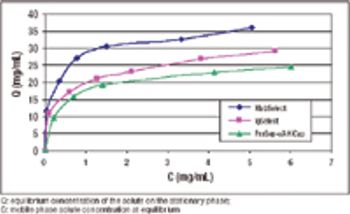
A stable alternative to Protein A chromatography.
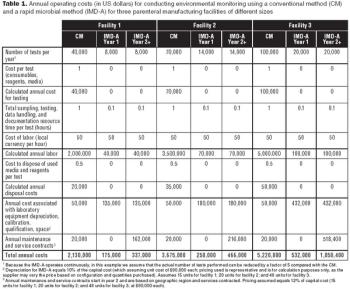
A case study implementing rapid microbiological methods.

A better method for trend analysis than CUSUM and control charts.
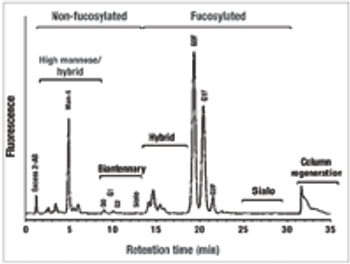
Select the best approach to determine critical quality attributes.

By following key strategies, companies can reduce the risk and increase the benefits of outsourcing analytical development and testing

Without a rigorous discussion of the pros and cons of QbD, its tremendous benefits will be lost.