
GE Healthcare's KUBio prebuilt modules were shipped from Germany to JHL Biotech in Wuhan, China.

GE Healthcare's KUBio prebuilt modules were shipped from Germany to JHL Biotech in Wuhan, China.

Univercells will integrate its single-use bioprocess with the Takeda vaccines production platform to allow local production.

BioPharm International eBooksVolume 28, Issue 14 Single-use technologies including polymer film bags, tubing, filter capsules, and appropriate connectors have become widely accepted in bioprocessing of proteins and vaccines.
The author explores a dual-supplier sourcing strategy for single-use products and its ability to mitigate business continuity risk.

Nexvet Biopharma, a veterinary biologics developer, secured a dedicated, cGMP biologics manufacturing facility in Tullamore, Ireland, and plans to invest in disposable technology.

Abenza acquired biopharmaceutical CDMO PacificGMP and expanded the company’s San Diego facility.
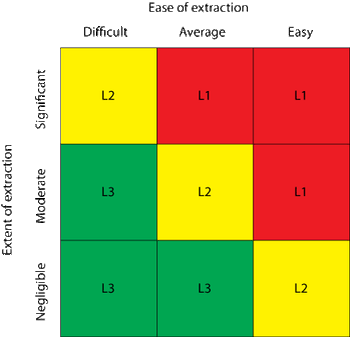
The author discusses the current best practices in technical qualification of single-use systems.
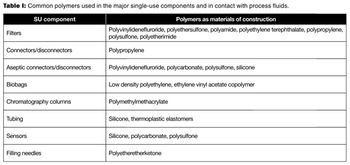
New single-use technologies and other filtration systems are beginning to address cost, throughput, and manufacturing footprint demands.

The partnership will help divert 840 tons of waste related to single-use products from landfills or incineration during the next year alone.

Manufacturers are producing new drugs and vaccines and clinical supplies faster and more efficiently through the development of standards and common practices for single-use technology systems.

The Mobius 2000-L single-use bioreactor from EMD Millipore offers configurable software, hardware, and single-use Flexware assemblies for suspension and adherent cell-culture applications.

The challenges and strategies of assessing and mitigating risk in biopharmaceutical manufacturing are discussed.

The use of single-use systems in downstream processing offers benefits in filtration and sampling and may reduce the risk of contamination.

Sartorius Stedim Biotech’s Sartoclear Dynamics is a clarification system designed to harvest mammalian cell cultures with high cell densities using single-use technology.
Single-use and modular technologies plus continuous manufacturing are increasingly important to biopharma scale-up and tech transfer.

Pall has agreed to be acquired by Danaher for $127.20 per share.

Integrating advances in facility design can meet differing and emerging bioprocessing needs.

This article presents first-hand perspectives from industry users to suppliers of single-use sensors.

Thermo Fisher Scientific adds Advanced Scientific’s custom single-use systems and equipment to its bioprocessing offerings.

Evaluating the assembly design process, manufacture, and use helps mitigate risk.

Single-use components aid efficiency in automated personalized therapy manufacturing.

The authors review efforts to limit polymer degradation without significantly impeding cell growth.

Biomanufacturing capacity expansion uses modular cleanroom design and single-use technologies.

The $200-million project will expand Amgen's single-use/disposable manufacturing capabilities.

New technologies facilitate enhanced single-use processes and procedures.