
Continuous countercurrent tangential chromatography (CCTC) is a column-free process that provides a scalable, disposable, and cost-saving alternative to column chromatography.

Continuous countercurrent tangential chromatography (CCTC) is a column-free process that provides a scalable, disposable, and cost-saving alternative to column chromatography.
Uwe Gottschalk, vice-president of purification technologies, Sartorius Stedim Biotech, discusses specific challenges in protein purification.

Choosing the right disposable components for your application.


Rita Peters, Editorial Director for Pharmaceutical Technology and BioPharm Inernational talks with Mani Krishnan, director of systems at EMD Millipore live at Interphex 2013 about single-use technology. In this interview they discuss areas of growth in single-use technology as well as reservations that some companies have to switching to single-use systems Krishnan identifies a few technology advances that are overcoming these objections as well as decision-making strategies for facilities considering a switch to a single-use system.

BioPharm International spoke with INTERPHEX 2013 conference-session presenters to gain insight on trends in facility and process design.
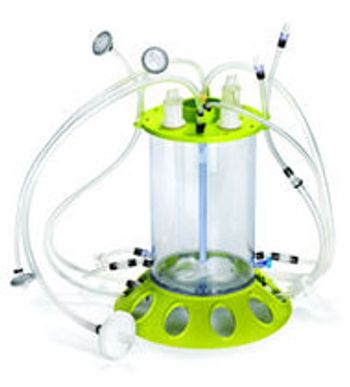
Review the process-design space and scalability of a single-use, stirred-tank bioreactor.

Jerold Martin considers the types of tubing available to the industry and how to make an informed selection.

Reduced carryover risk and close attention to particulate control result in greater patient safety through the use of single-use systems.

BioPlan's Annual Report shows continued growth in the use of single-use technology.
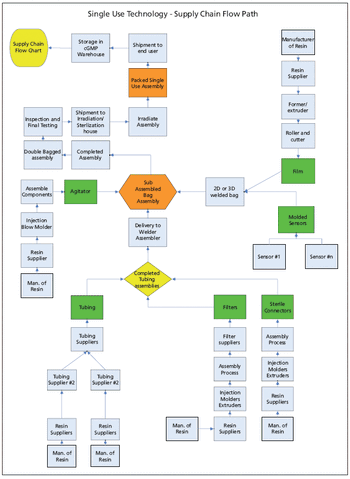
The authors suggest techniques for mitigating risk and securing the supply chain for single-use components used in biopharmaceutical manufacturing.
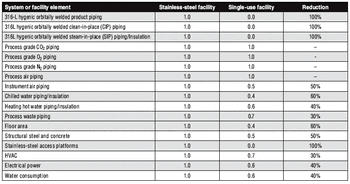
The move to single-use manufacturing has prompted a paradigm shift in facility design.

The authors describe the growth characteristics of human mesenchymal stem cells cultured in a stirred-tank bioreactor.

Kevin Ott, executive director of the Bio-Process Systems Alliance, discusses the latest trends and issues surrounding single-use technologies and the role of his association.

What the industry's future holds and what needs to be done to get there.
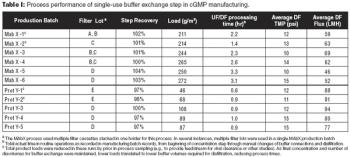
This case study describes the process used to transition from a multi-use system to single-use tangential flow filtration for performing final buffer exchange steps.

Industry experts from GE Healthcare, Tarpon Biosystems, Polybatics, Merck Millipore, and Repligen discuss the challenges of adapting disposable technology to the chromatography process.

This article describes best practices for implementing a single-use process train at a bioproduction facility.
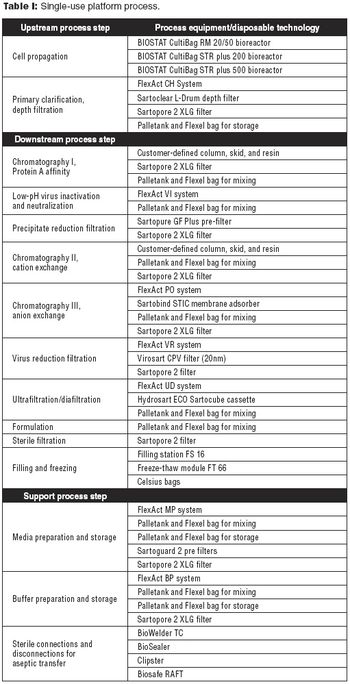
The combination of single-use platform technology with modular facility construction is a template for flexible manufacturing.

A brief case study of a facility-fit analysis provides insight into how to adjust capacity when moving from clinical-to commercial-scale production.

The authors explain why Catalent decided to transition from stainless steel to single-use systems.

GE Healthcare Life Sciences' ReadyToProcess platform aims to streamline bioprocessing.
Insights on single-use systems implementation and exploitation in biopharmaceutical manufacturing and processing, based on a QbD approach.

The author considers the types of tubing available to the industry and how to make an informed selection.

This article discusses the evaluation of a novel single-use fluidized bed centrifuge for harvesting of antibodies.