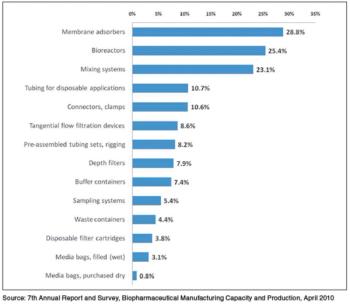
Although single-use systems are widely used in upstream unit operations, their acceptance in downstream processes has been slow.

Although single-use systems are widely used in upstream unit operations, their acceptance in downstream processes has been slow.

How a Big Pharma company tackled the move to disposable bioreactors.

New Brunswick Scientific, an Eppendorf Company (Edison, NJ), and Pall Corporation (Port Washington, NY) have entered into a product development and marketing agreement to create and supply new disposable bioreactor systems.

Humacyte, Inc. (Research Triangle Park, NC) and Xcellerex, Inc. (Marlborough, MA) have entered into an initial strategic collaboration for Xcellerex to develop a manufacturing process that will enable the production of Humacyte?s lead regenerative medicine product using Xcellerex?s XDR single-use bioreactor system in its FlexFactory.

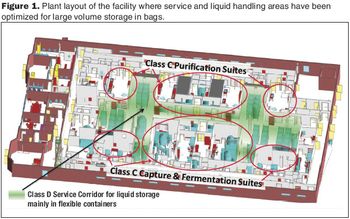
How this Big Pharma company successfully implemented disposable technologies in its manufacturing plant.

Suppliers, manufacturers, and governments must work together to plan how best to develop and deploy disposable systems for emergency response.
A case study compares capital costs, operating expenses, and NPV for a new MAb plant.

A case study assesses freezing time and physical robustness under stress.
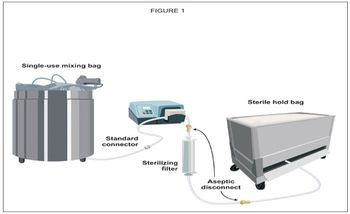
Single-use connections can help drug manufacturers maximize efficiency in every step of the manufacturing process.
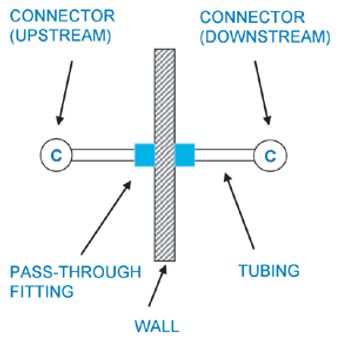
Many factors must be considered when choosing a sterile connector for a given process.
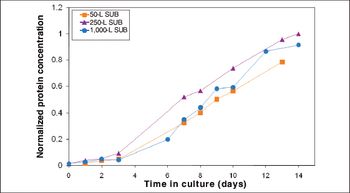
Process performance was comparable across all scales, and fiber optic sensors appeared interchangeable with conventional probes.
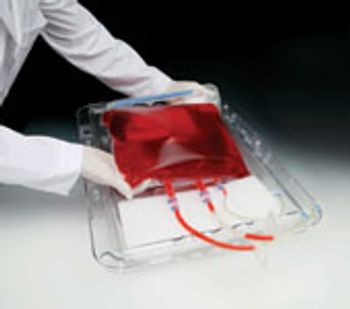
Genentech's evaluation of single-use technologies for bulk freeze-thaw, storage, and transportation.

To assess current trends in single-use bioprocessing equipment, BioPharm International turned to John Boehm, bioprocessing business unit manager, Colder Products Company; Mandar Dixit, head of product management?filtration technologies, Sartorius Stedim Biotech; Geoff Hodge, managing director of process technology Xcellerex, Inc.; Günter Jagschies, senior director of strategic customer relations, life sciences, biotechnologies, GE Healthcare; Mani Krishnan, director of product management, Mobius single-use technologies, Millipore Corporation; and Jerry Martin, senior vice president of scientific affairs, Pall Life Sciences, and board chairman and technology chair at BPSA.

To assess current trends in laboratory equipment, BioPharm International turned to Francis Bach, North American sales director, Asahi Kasei TechniKrom, Inc.; Erika Lapinskas, PhD, product manager, Sartorius Stedim Biotech; Jeffrey R. Mazzeo, PhD, biopharmaceutical business director, Waters Corporation; Amber Ratcliff, product manager, analytical sensors, Hamilton Company; and Josh Silverstone, marketing director, Millipore Corp.

Single-use technologies can be configured and installed fairly quickly, but are they ready to handle the urgency and scale of a pandemic?

What end users think about single-use systems.
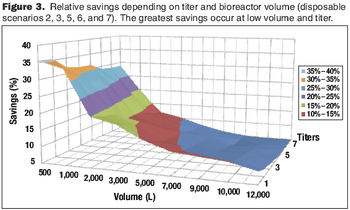
Cost modeling provides valuable insights to support strategic decision-making when implementing disposable technologies.


How to choose a disposable mixing system that fits your particular needs.
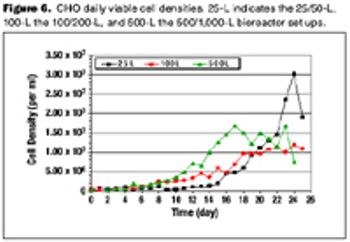
Are disposable bioreactors effective for cell culture?
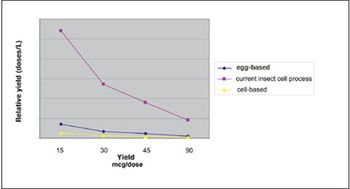
With virus-based production, vaccines can be available in 10-12 weeks.
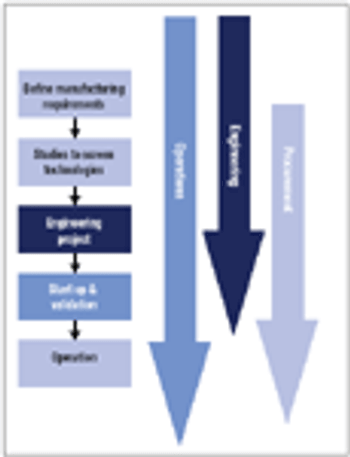
In new disposables projects, it is critical that engineering, procurement, and operations groups work together early on to manage supply chain risk.

A major problem in personalized medicine has been scale-up.

Millipore Corporation (Billerica, MA) and Applikon Biotechnology BV (Schiedam, The Netherlands) have announced an agreement to co-develop and distribute disposable bioreactor systems for biopharmaceutical applications.

Transitioning from stainless steel technology to disposable technology in bioprocessing.