
More published data and initial regulatory approvals are needed to drive adoption of continuous bio-manufacturing.

More published data and initial regulatory approvals are needed to drive adoption of continuous bio-manufacturing.

The company unveiled three new products to support single-use biomanufacturing and won an award for best technological innovation at INTERPHEX 2018.

The company is launching a new pre-fabricated, ready-to-run manufacturing facility that is expected to significantly decrease the production timeline for viral vector-based therapeutics.

The biopharmaceutical firm has chosen Rhode Island as the site of its next-generation biomanufacturing plant, which will offer flexibility, speed, and efficiency.

Early adopters are benefiting from lower costs and increased productivity.
Developments and investments in single-use systems advance upstream biomanufacturing.

The company plans to install 4000-L disposable bioreactors from ABEC at its new commercial manufacturing facility in Wuxi city, China.

The contract development and manufacturing organization has expanded biologics capacity at its facility in Berkeley, CA.

The new training center, the Jefferson Institute for Bioprocessing, will prepare engineering students and industry professionals for the field of biologics manufacturing.

The company has expanded its single-use manufacturing capabilities at its facility in Fermoy, Ireland.

The Flexicon PF7 peristaltic tabletop aseptic liquid-filling machine from Watson-Marlow Fluid Technology Group is suitable for GMP-regulated biotechnology and pharmaceutical operations.

Layout and supply details must be considered when implementing a fully disposable biopharmaceutical manufacturing process.
Single-use technologies are starting to gain ground as capacity needs change, but industrywide adoption remains low.

BioPharm International asked Andrew Bulpin, head of Process Solutions at MilliporeSigma, about the end-of-life options for disposable components of biopharmaceutical single-use systems.

Clover Biopharmaceuticals will use GE's FlexFactory biomanufacturing platform with single-use bioreactors to produce innovative and biosimilar fusion protein products in China.

GE Healthcare will equip Cellular Biomedicine Group's cell therapy manufacturing facility in Shanghai, China, with its FlexFactory single-use platform, designed to speed up cell therapy manufacturing timelines.

Sartorius Stedim Biotech will equip Abzena’s contract development and manufacturing facilities in Bristol, PA, and San Diego, CA, with single-use equipment and scale-up technologies.

Proper selection and installation optimizes fluid system performance.
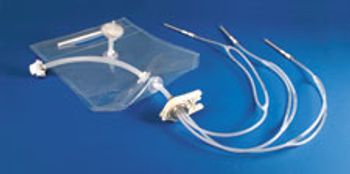
Sterile-molded filling assemblies from AdvantaPure are suited for single-use vial and syringe filling.

MilliporeSigma will collaborate with IPS and G-CON to offer end-to-end, turnkey, modular MAb manufacturing.

The Quattroflow QF10k size pump from PSG, a Dover company, has been added to its line of quaternary, four-piston diaphragm pumps.

A roundtable Q&A with biopharma executives elucidates the challenges posed by single-use bioreactor bags in contributing to extractables and leachables in the biomanufacturing process.

SciLog Select Go Single-Use Assemblies from Parker Hannifin uses an assortment of validated parts and assemblies to build needed devices for biopharmaceutical manufacturing.

The company’s new modules offer scalable single-pass diafiltration and were exclusively showcased during its Leadership Forum series in Westborough, MA.
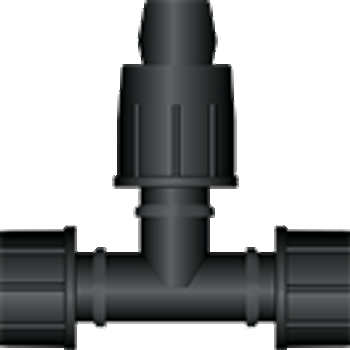
Connectors are a critical element in the process optimization of single-use bioprocessing systems.