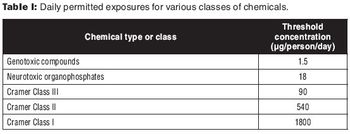
This article is the second in a two-part series on extractables and leachables.

This article is the second in a two-part series on extractables and leachables.

This article outlines methods, validation standards, and documentation of sterilization of single-use products using gamma irradiation.

This month, we rewind to "Separations Technology Outlook, Part II: Improved Recovery and Greater Purity."

The author describes automated equipment that uses functionally closed disposables.

Readers react to the economic turmoil of the past year and look longingly forward to 2012.
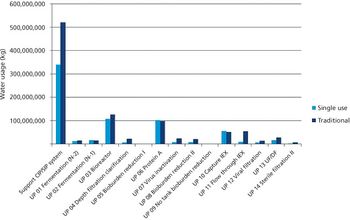
The authors compare the environmental impact of monoclonal antibody production using fixed-in-place processing and single-use systems.
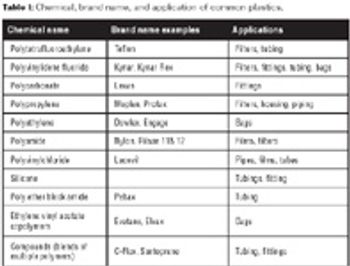
An overview of applications for disposable components and important property considerations.
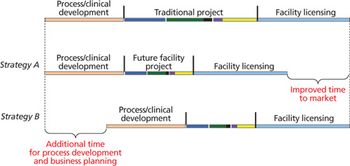
With careful analysis to mitigate risk, disposable technology and process closure can enable adaptable designs and reduced costs.
New technology is designed to improve production efficiency by taking advantage of the properties of single-use bags.

Single-use manufacturing may seem like a new trend, but it has actually been around for almost 30 years, beginning in the early 1980s when filter manufacturers began to make small process-scale plastic filter capsules to replace "junior" size stainless-filter housing assemblies.
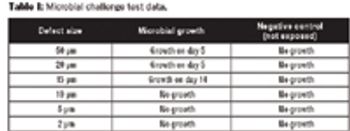
Defects as small as 10 μm can be detected without compromising product cleanliness using helium integrity testing.

In this quarter's column, highlights from the IBC Single-use Applications meeting, the PDA Single-use Workshop, and the BioProcess systems Alliance International Single-Use Summit are presented.

Single-use systems continue to gain traction among biomanufacturers, especially CMOs.

Developing a quality agreement template for single-use systems.
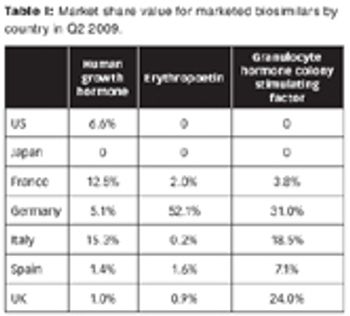
The market landscape for biosimilars is in flux, with limited penetration now, but with the potential for growth for those who can navigate the market. Plus: A SWOT analysis of biosimilars by Anjan Selz.

The author looks at strategies to minimize particle levels in the finished product when using single-use technologies downstream of final capabilities.
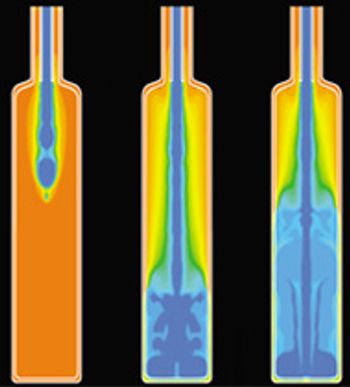
The authors give special consideration factors affecting blow–fill–seal technology.

Single-use conference roundup.
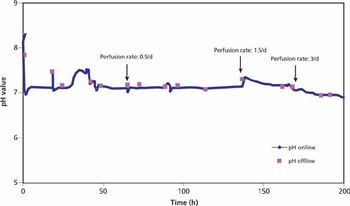
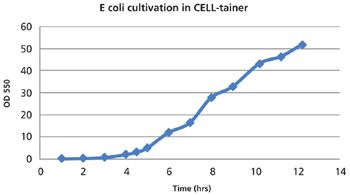
The authors discuss the use of single-use bioreactors.

Although some aspects of single-use components can be standardized, it is unlikely that any materials or design features will become a commodity
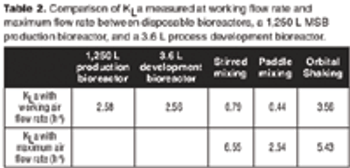
A case study to compare the performances of several types of mixing in disposable bags with stainless steel bioreactors.
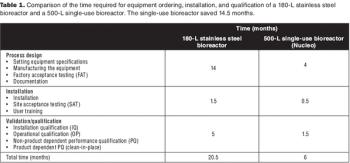
Sanofi Pasteur's disposables implementation plan is part of a larger evaluation of technology innovation. Here's how they approach it.

ATMI, Inc. has acquired the Belgian biotechnology firm Artelis SA, which offers technologies for cell culture research and manufacturing scale-up.

A case study evaluates the performance, control of operations, productivity, and cost savings of a single-use system.