
ABEC increases the maximum capacity of its Custom Single Run Bioreactors to 4000 L, doubling the industry standard.

ABEC increases the maximum capacity of its Custom Single Run Bioreactors to 4000 L, doubling the industry standard.
While the measurement of the toxicity of leachables is not always a required parameter, the information collected during these studies could inform future bioprocessing runs.

Single-use bags containing toxic or hazardous materials required special handling.

Quattroflow’s EZ-Set Pump Chamber Replacing System for its single-use pumps allows users to change chambers quickly by hand.

Avid, a wholly owned subsidiary of Peregrine Pharmaceuticals, will upgrade its Myford, California clinical and commercial manufacturing facility with multiple Mobius 2000-L single-use bioreactors from MilliporeSigma, the companies announced on May 1, 2017.

MilliporeSigma’s new high-area cartridge filters and single-use capsules are suitable for feed streams with high levels of particulates.

Tony Pidgeon, process technology director at Patheon discusses challenges associated with and recent innovations in the fill/finish process for biopharmaceuticals.

The facility in Britley will now manufacture the company’s automated systems, enabling the company to better serve European markets and shorten the supply chain.

The Mobius MyWay portfolio offers off-the-shelf and configured-to-order single-use assemblies.

The process control and automation requirements of single-use systems differ from those of stainless-steel equipment.

The decision to use disposable bioreactors is now driven by commercial rather than technological considerations.

To prevent failure during lengthy use, tube life should be monitored and a preventive maintenance program enacted.

Extraction studies demonstrate approaches for evaluating single-use bio-pharmaceutical manufacturing materials.

SGS invested in test equipment for analyzing extractables and leachables at its New Jersey laboratory.

The authors describe the development and validation of a highly sensitive point-of-use pressure decay test.

By working together to harmonize the highly variable steps within the biopharmaceutical manufacturing process, both end users and suppliers are making strides toward the efficiency and integrity of single-use technology.

Experts discuss recent advances in cell viability testing methods in bioreactors.

A PESU membrane is now available for Sartorius Stedim Biotech Sartocon benchtop and production-scale filtration assemblies.

R-Pharm Group, a private Russian pharmaceutical company, has opened a biopharmaceutical production plant in Yaroslavl, Russia, to produce biologics and biosimilars to treat autoimmune diseases and cancer. The facility, which has a line capacity is 2,000 L of cell culture per production cycle, is ready to be validated to US and Russia FDA and GMP requirements, the company reports.

GE Healthcare’s GE BioPark Cork will hold four KUBio manufacturing facilities; GE will also collaborate with NIBRT for biopharmaceutical training.

GE and Sealed Air extend their long-term collaboration to develop single-use films purposefully constructed for bioprocessing applications.

Industry experts discuss the challenges of using single-use systems in biopharma manufacturing.
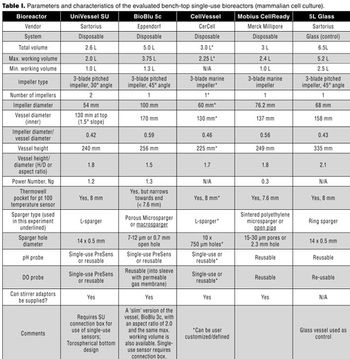
The authors present a case study in which four single-use vessels were fitted to an existing bioreactor system.

Alvotech prepares for commercial biosimilar production in new facility with single-use bioreactors in Reykjavik, Iceland.

Advances in single-use systems, consumables, and continuous manufacturing show steady progress.