
Fed-batch processes were scaled up from traditional bench-scale bioreactors to large-scale single-use systems.

Fed-batch processes were scaled up from traditional bench-scale bioreactors to large-scale single-use systems.

The EZ-Fit Filtration Unit, a ready-to-use, disposable filtration device for bioburden testing from EMD Millipore, the Life Science division of Merck KGaA, increases reliability and streamlines workflows.
Experts discuss the future of modular manufacturing and the challenges that biopharma manufacturers face in facility design

Attendees at the Bio-Process Systems Alliance annual summit discuss the benefits of single-use technology in biopharmaceutical manufacturing.

Innovative polyethylene film sets new benchmark
Suppliers see challenges to the adoption of single-use technologies for downstream processing as opportunities.

Progress is being made in the development of harmonized best practices for single-use systems.

Integrated Single-Use Sterile and SIP Connector Provide Flexibility

Grace's ProVance disposable Protein A chromatography column is designed for downstream purification.

For single-use systems, supply chain excellence requires a commitment to problem solving across organization boundaries.

Research uncovers trends and factors affecting the pre-packed disposable chromatography columns market for downstream bioprocessing.

Experts discuss the advances in single-use technology for downstream bioprocessing applications.

Adopting single-use systems has implications for manufacturing execution systems, process data-analytics tools, and scheduling

A novel approach to sterile drug product manufacturing uses a single-use assembly in a multi-product final filling suite with isolator technology.

An environmental study of single-use process technology for biopharmaceutical manufacturing offers a comprehensive examination of environmental impacts across the full process train using lifecycle assessment.

Plastic pump chamber is a cost-effective alternative for biopharmaceutical applications.

ASI Life Sciences introduced the DHX Disposable Heat Exchanger and the imPULSE MDS mixing, docking, and shipping system for single-use biopharmaceutical processes.

Research uncovers trends and factors affecting the pre-packed disposable chromatography columns market for downstream bioprocessing.

Experts discuss the advances in single-use technology for downstream bioprocessing applications.
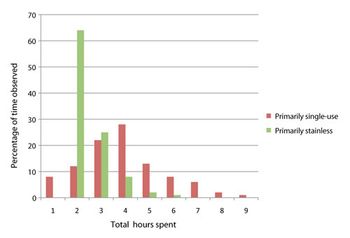
Adopting single-use systems has implications for manufacturing execution systems (MES), process data-analytics tools, and scheduling. Enterprise resource planning and MES must be integrated using standards such as ISA-95. Master and electronic batch records must be designed to capture the details of single-use component assembly to enable traceability.

ALpHA G Capsule Filter for Single-Use Systems

For single-use systems, supply chain excellence requires a commitment to problem solving across organization boundaries.
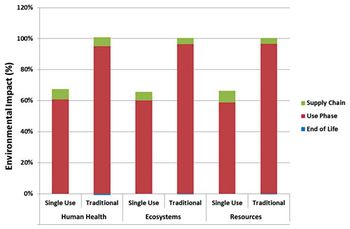
An environmental study of single-use process technology for biopharmaceutical manufacturing offers a comprehensive examination of environmental impacts across the full process train using lifecycle assessment.

A novel approach to sterile drug product manufacturing that uses a single-use assembly in a multi-product final filling suite with isolator technology offers benefits of efficiency and flexibility.

Studies show diminished cell growth properties associated with some biocontainer or bioreactor films.