
The comparative research approach may be preferable to price controls in the guise of government negotiations for the Medicare drug benefit, coverage denials, and limits on access to new technologies.

The comparative research approach may be preferable to price controls in the guise of government negotiations for the Medicare drug benefit, coverage denials, and limits on access to new technologies.

A staged approach to limits should embrace future capabilities.

The industry needs to open up to validation failures.

US Food and Drug Administration's Division of Biologic Oncology Products has approved two new biologics license application (BLA) supplements expanding the approval of Genentech's Herceptin (trastuzumab) for the treatment of breast cancer.

The FDA is under attack from all sides. Many influential members of Congress either don't trust the agency to monitor the industry appropriately, or have found it politically expedient to keep sounding alarms about inadequate oversight of food and drug safety and clinical research. The good news is that there seems to be a growing consensus that FDA needs a major infusion of cash to regain its stature as an effective science-based regulatory agency.
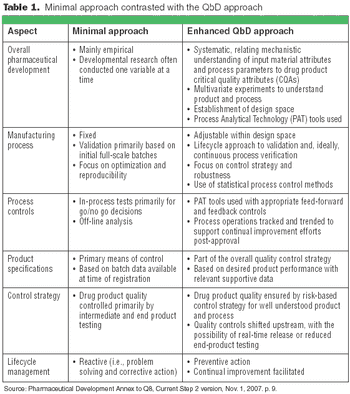
The principles of QbD can be applied to biotech development and manufacturing to help resolve many common issues. QbD scientifically provides a greater understanding of the complex relationships among product quality attributes, the manufacturing process, and clinical safety and efficacy by determining the various permutations of critical input variables that will keep the product within specification.
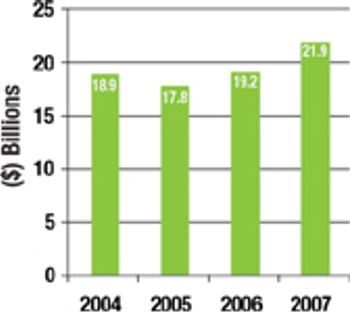
The current overcapacity situation in the bio/pharmaceutical industry is a reminder that CMOs need to come up with business models and value propositions that are based on more than just selling capacity.

Recent problems with food and pharmaceutical ingredients sourced from China highlight a major disadvantage of our complex international supply chains for food and drug ingredients. A global supply chain offers more opportunities for accidental contamination as well as intentional adulteration and counterfeiting. Sticking to minimal requirements may not be enough.
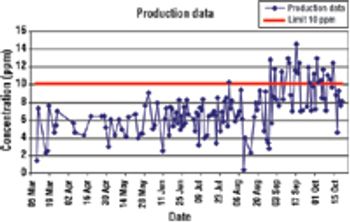
CUSUM charts are easy to create in Excel and can reveal when a change occurred.

In its effort to ensure that consistent definitions of terminology are being applied by all constituents of the ICH, the FDA has released a new guidance, E15, containing definitions of key terms in the discipline of pharmacogenomics and pharmacogenetics.
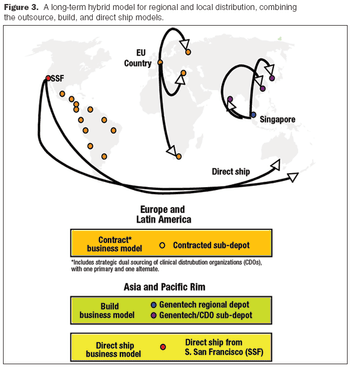
A decision-criteria matrix and cost models helped pinpoint the best distribution approach for the short- and long-term.

There are several considerations to keep in mind when auditing an outsourcing provider.
The outsourced service provider should be considered an extension of your own laboratory.

After a strategic evaluation of what activities to outsource, sponsor companies should follow key guidelines for selecting and auditing a provider and preparing quality agreements.
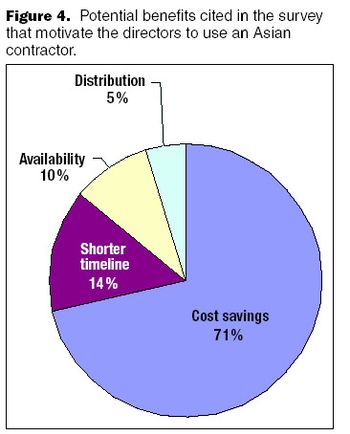
When a biopharmaceutical company pursues an outsourcing strategy, the choice of a contractor is a critical and strategic decision.

The book is a useful, comprehensive, and truly an excellent reference source of biopharmaceutical information.

Engaging executive leadership in the quality process is the key to compliance success.

A case study investigated the root cause of failures in sterile filtration by evaluating the effects and interactions of four operating parameters.

Did the 2005 Patents Act engender a Western intellectual property rights culture in the country?

The heparin safety crisis puts a spotlight on manufacturing processes and regulatory oversight.

Now is a good time for companies to know their suppliers well.

The supply base for preclinical and clinical development services continues to expand in China.

India is restructuring its regulation of biopharmaceuticals to help the country's industry compete internationally.

More informed submissions may lead to regulatory flexibility for postapproval changes.
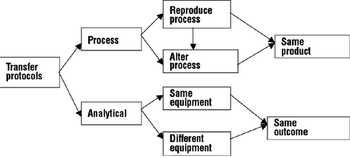
A comprehensive process and analytical transfer package can speed up your product's time to market and save costs.