
Use Lean techniques to improve manufacturing compliance

Use Lean techniques to improve manufacturing compliance

Without a rigorous discussion of the pros and cons of QbD, its tremendous benefits will be lost.

How to optimize facility utilization and improve plant performance.

Avoiding healthcare reform is not the best option for the pharmaceutical industry.

Why the industry needs to review its traditional approach to facility validation

This article reviews some of the commonly used approaches for process monitoring as well as the evolution of process monitoring in the Quality by Design (QbD) paradigm.

Authorities are pushing for CE; manufacturers prefer to focus on value.

ImClone Systems, Inc. (Branchburg, NJ), and Bristol-Myers Squibb (BMS, New York, NY) voluntarily recalled 13 lots of their cancer drug Erbitux (cetuximab) after a report from a doctor?s office about a leaking cap on a vial of the biologic.
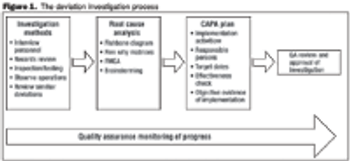
Follow a risk-based approach to maintain a state of control.

The FDA is poised to gain more authority and resources to ensure product quality.

The 45 comments submitted raised concerns about legacy products and ongoing process monitoring.
A method to evaluate the relative cleanability of new products.

At some point, will heavy investments in large, stainless-steel based facilities become a burden to US companies?

Broader transparency in product prices and payments to researchers aim to curb conflicts of interest and rationalize drug expenditures.

With counterfeit and adulterated medicines posing an increasing risk to patients in the United States and worldwide, the US Pharmacopeial (USP, Rockville, MD) Convention announced on February 4, new standards for two widely used drug products that have been involved in episodes of adulteration resulting in patient deaths.

How change plays out will depend not only on the new Whitehouse, but on pharma leaders' ability to adapt to changing times.

QbD for QA? Try a two-step approach
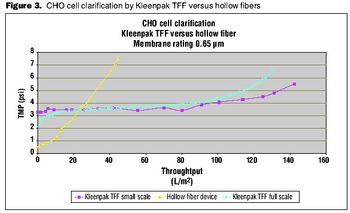
Choosing the right tools to enhance the process.

With cuts in healthcare, biotech drugs are under scrutiny.

Understanding the relationship between the process and CQAs.

FDA aims to regain public confidence in 2009.
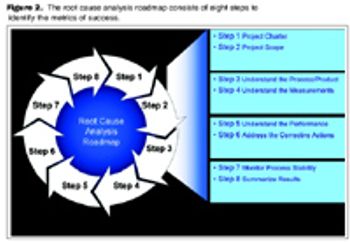
Optimize time and cost of product development by managing risk.

Looking ahead at the biopharmaceutical industry into 2009.

QbD can help satisfy FDA and EMEA requirements.

The development of a skilled labor force is essential for an expanding biopharmaceutical industry.