
What football and bioprocessing both have in common is that in both cases, success is a minimum requirement.

What football and bioprocessing both have in common is that in both cases, success is a minimum requirement.
First in a three-part series that discusses the complexities of QbD implementation in biotech product development.

The focus on the design space will lead to a new workspace, and will affect staff in the development, manufacturing, and quality functions.
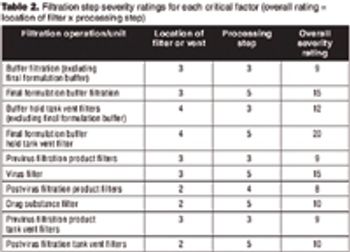
Conducting a FMEA analysis is a good first step in a risk-based approach for determining the need for a filter integrity test.

Heightened attention to product safety issues is slowing the approval process for new therapies.
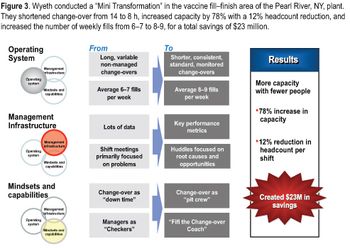
To make lasting change, you must first win the hearts and minds of the people on the shop floor.
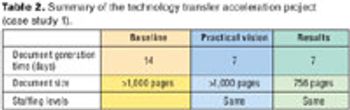
A step-by-step approach is essential for successful implementation.
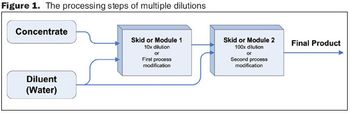
Automated in-line dilution can help solve capacity, financial, and quality concerns that biopharmaceutical manufacturing plants may be facing.

It seems clear that insuring the roughly 46 million Americans who are now uninsured will increase drug sales.

Stiffer enforcement of quality standards aims to restore public confidence in agency actions.

To assess current trends in cleanrooms and engineering & facilities, BioPharm International turned to Parrish Galliher, founder and chief technology officer, Xcellerex, Inc.; Jim Maslowski, owner, PDC Aseptic Filling Systems; Morgan Polen, vice president, application technology, Lighthouse Worldwide Solutions; and Benoît Verjans, commercial director, Aseptic Technologies.

If risk assessments only identify "the usual suspects," the process will not add much value.

The FDA is encouraging manufacturers to invest in research and development for new vaccines and therapeutics to combat third-world diseases.

ICH Q9 encourages companies to apply the concept of quality risk management. Easier said than done.

A better method for trend analysis than CUSUM and control charts.
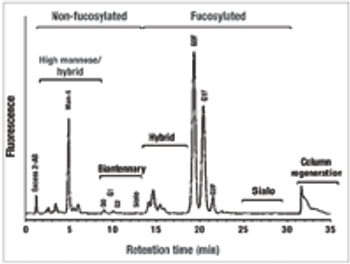
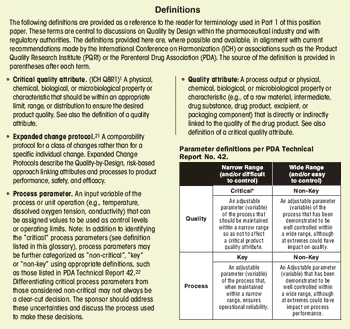
Select the best approach to determine critical quality attributes.

Perhaps the best way to regulate drugs is to regulate them not conservatively or liberally, but effectively.
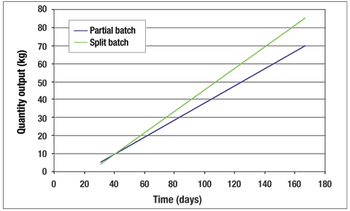
A case study in capturing indirect costs and benefits.

Enabling site-wide process efficiency.

Pressures to reduce healthcare spending generates proposals to spur competition, cut costs.

Companies often wait for a critical mass before adopting new technologies. But if no one takes the risk, critical mass will never be reached.

A culture of quality that emphasizes business objectives, risk management, and the informed application of technology can improve compliance.
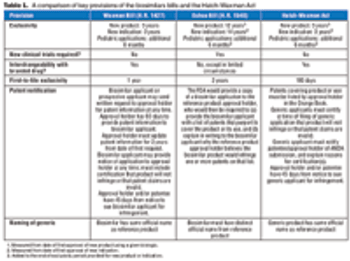
The introduction of two rival bills has intensified the long-simmering debate on biosimilars regulation in the US.

The truth is, we should have been afraid of H1N1, because the threat of a flu pandemic is real.

Biopharmaceutical companies must follow an active approach to managing their supply chains.