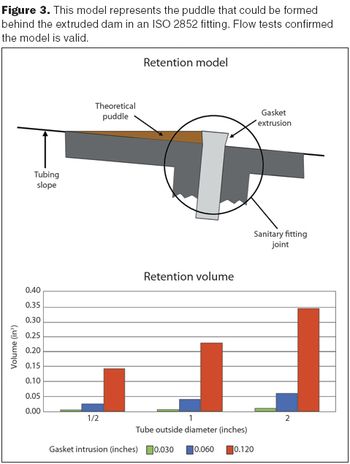
How to control compressive loads on seal materials.

How to control compressive loads on seal materials.

New R&D models must be tried, but it will take time to see if they work. In the meantime, a new kind of threat is on the horizon: biobetters.

Broader benefits and biosimilars will offset hefty fees and discounts while preserving R&D incentives.

This practicum combines business elements with scientific concepts.

Computational fluid dynamics can resolve performance problems.

It is important that all stages of the audit be given an equal measure of attention.
A process harmonization assessment can aid in smooth technology transfer by comparing data across equipment and sites.

Given today's challenges, traditional strategies were bound to change. Now, there is one more big threat (or opportunity?) on the horizon.
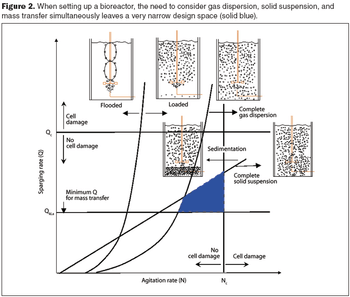
Computational fluid dynamics is a powerful tool to optimize processes.

The FDA is expanding postmarketing safety requirements, despite limited resources to manage these added responsibilities.

Characterizing the higher order structure (HOS) of protein drugs increases manufacturers' understanding of stability and batch-to-batch variability, and may make it possible to link variants or aggregates to safety and efficacy. Yet at the January 24 CMC Strategy Forum in Washington, DC, regulators expressed concern that methods to characterize the three-dimensional structure of proteins are not routinely applied to biotechnology products.

An effective CAPA plan provides a mechanism for responding to the unexpected.

The new Sentinel system aims to expand access to data on medical product safety and patient effects.

As a key element in the peptide production process, quality should be built into every step.

Sharing information is a critical part of security-whether we're protecting travelers from bombs or patients from adulterated medicines.

Trouble at Genzyme and with flu vaccine production illustrates the challenges in producing safe and potent biologics.

One might look at QbD's plodding growth and conclude that it is never going to make it to graduation.
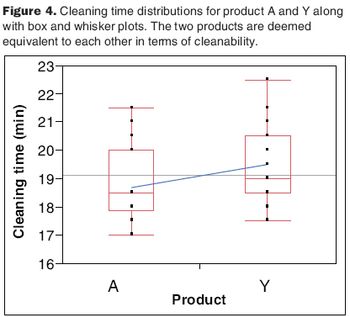
The two-one-sided t-test compares the equivalency of two data sets.

How will implementing Quality by Design strategies affect your compliance status?

Demand for new vaccines and therapies in 2010 will be offset by concerns about drug prices and product safety.

The nimbleness of biotechs makes them well suited to implementing QbD. Here's how to get started.

Regulatory flexibility can make continuous improvement possible.

QbD has always embraced the notion that companies could make certain process changes without regulatory oversight.
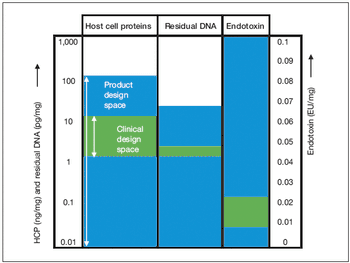
Key considerations for defining your overall control strategy.
