
Regulatory agencies have evolved along with the biotechnology industry to define quality standards.

Regulatory agencies have evolved along with the biotechnology industry to define quality standards.

Favrille, a San Diego-based biopharmaceutical company, is one of a handful of firms on the forefront of personalized medicine. Because personalized treatment is tailored to an individual's biology, it has the potential to be far more effective than current approaches to disease management.

It became a strategic imperative to find a better, more efficient way to manufacture our products. To continue with the status quo was untenable.
Federal regulation of biologics began more than 100 years ago with the enactment of the Biologics Control Act of 1902. That act required licensing of establishments to manufacture and sell vaccines, sera, antitoxins, and other similar products in order to prevent deaths from contaminated vaccines, as had recently occurred with the diphtheria antitoxin.

Multivariate data analysis can help biotech manufacturers deepen their process understanding.
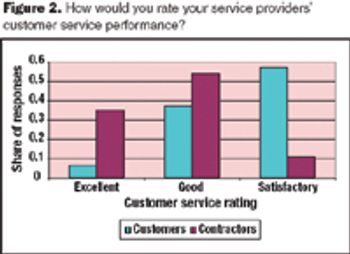
Meeting service levels is a major challenge for pharmaceutical services providers because the requirements of their client base vary widely.

The new legislation authorizes the use of fee revenues for drug safety oversight and assessment throughout a product's lifecycle.


In light of these recent decisions, patent holders must devise new strategies for patent enforcement and licensing.


From the earliest days of the biotechnology industry, companies have grappled with the complexities of making innovative biopharmaceuticals on a large scale. Success in manufacturing begins with process science, since biotech production requires perfection in maintaining living organisms in a sterile environment under controlled physiological conditions. But unless companies can solve the challenge of planning for and managing manufacturing capacity, they will not be able to achieve the full potential of promising biotech products.

The US Food and Drug Administration's Nanotechnology Task Force (www.fda.gov/nanotechnology/ nano_tf.html) has released a report recommending the agency develop guidelines and take other steps to address the benefits and risks of products, including drugs and medical technology, that use nanotechnology.

The US Food and Drug Administration (FDA, Rockville, MD, www.fda.gov) issued a revised draft guidance on July 20 to help ensure that the safety, purity, and potency of biologics products is not compromised as a result of innovative, flexible manufacturing arrangements.
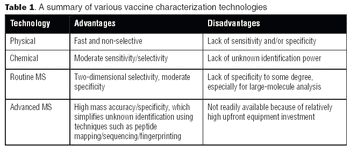
With the advent of high-resolution mass spectrometers and highly sensitive MS instruments, vaccine characterization has entered a new phase.

Any endpoint considered appropriate to support approval, whether a surrogate or a clinical endpoint, must be supported by substantial evidence of effectiveness.
The corruption of China's food and drug sectors is not limited to one man. Likewise, real reform will require the efforts of many.

Understanding the end-to-end management of chemistry, manufacturing, and controls (CMC) resources provides the opportunity to enhance long-term planning, leverage development options, manage resource trade offs, and track progress against plans. The goal is to improve the pharmaceutical development process to deliver the pipeline. This article provides an overview of the organizational structure of Process Research and Development (PR&D) and the CMC teams at Genentech; the alignment of resources based on CMC contracts, process development activity maps and project resource plans; and the business economic analysis for evaluating development options.

The main testing and regulatory provisions of the FOB legislation reflect multiple trade-offs between the demands of innovators and generics firms.

The US Pharmacopeia (Rockville, MD, USP, www.usp.org) recently announced that the implementation period for its USP–NF general notices statement requiring all manufacturers to conform to recently revised residual solvent standards in General Chapter <467> has been extended from July 1, 2007 to July 1, 2008.

The US FDA (Rockville, MD, www.fda.gov) recently announced the availability of a draft guidance, entitled, Q10 Pharmaceutical Quality System.

In late June and early July, the US Congress moved forward on three important bills affecting the biopharmaceutical industry, related to follow-on biologics, the Prescription Drug User Fee Act (PDUFA), and the 2008 FDA budget.

In the April issue of BioPharm International, the article "BioPharmaceutical Operations Roadmap," provided a summary of the key industry gaps executives would like to close in the next 10 years. These goals came to my mind while I was attending Interphex this April in New York.

Now equipment makers are designing new systems that can be deployed rapidly, are less costly to build, and require less water and power to operate.

Quality guidelines are only as good as their implementation.
