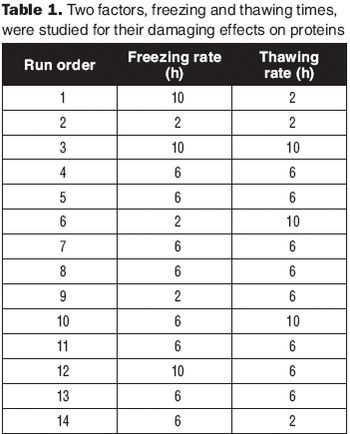
Apply a DoE strategy to test several formulations in parallel.

Apply a DoE strategy to test several formulations in parallel.

Changes on Capital Hill create uncertainty for healthcare reform, drug regulation, and biomedical research.

Evaluate and communicate risk to stakeholders.

Best practices to strengthen supplier quality management.

The pathway for biosimilar approval in the US has been set. But are US patients too far behind Europe?
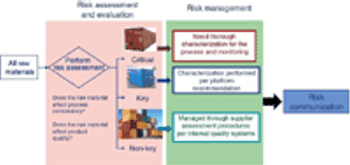
Adequate characterization of materials protects product quality.

Comparative effectiveness poses challenges for drug manufacturers.

How should the industry educate the public about product quality?

A new strategy to streamline vaccine development and oversight.

Industry and regulators disagree over noncritical parameters.

Too many REMS cause headaches for doctors and the industry.
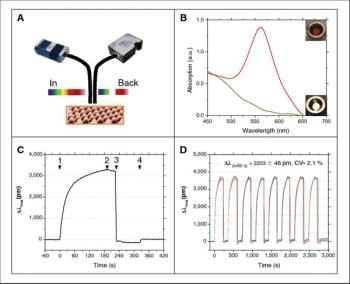
Use it label-free, or add labels to detect contaminants in solution.

What small biotechs need to know about quality management systems.

An in-depth analysis of the patent provisions of the new legislation.

The third holy grail of biosimilars: interchangeability.
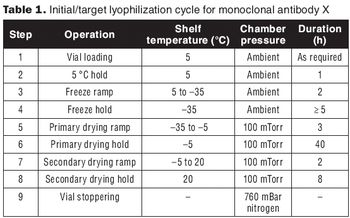
Develop a relevant design space without full factorial DoE.

EMa and FDA are seeking potential candidate companies for a joint GMP inspection pilot program.

The US Food and Drug Administration has designated XOMA 052, an antibody to interleukin-1 beta manufactured by XOMA, Ltd. (Berkley, CA), an orphan drug for the treatment of Behcet's disease.

Regulatory relief requires that regulators trust companies to know what they are doing, and to do it-consistently.

Plant closures, product recalls prompt FDA re-evaluation of GMP enforcement efforts.
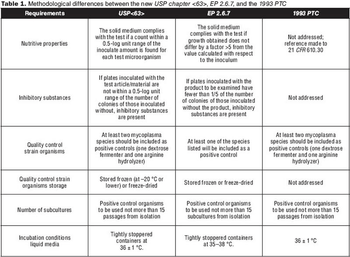
What you need to know about USP chapter <63>.
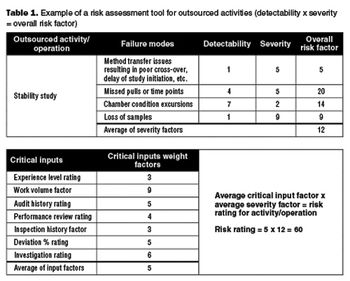
Apply risk management principles to monitor outsourced activities.
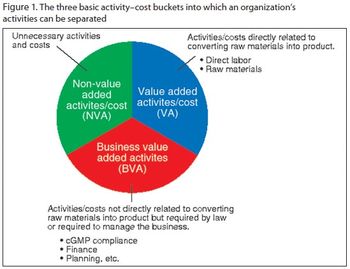
By identifying and eliminating non-value-added activities, drug manufacturers can avoid falling into the same cost-traps in the future.

A single standard should apply to all comparability exercises for biologics, be they for biosimilars or manufacturing changes.

USP is advancing efforts to develop a guidance for evaluating bioassays.