
This month, we rewind to "Separations Technology Outlook, Part II: Improved Recovery and Greater Purity."

This month, we rewind to "Separations Technology Outlook, Part II: Improved Recovery and Greater Purity."

Greater emphasis on focus and efficiency for companies as market demands value in 2012.

In a Warning Letter, FDA cited ?significant violations? of CGMP regulations, including several repeat observations, at three Novartis facilities. The violations included failure to prevent microbiological contamination of sterile drug products, failure to investigate out-of-specification batches, failure to clean and maintain equipment, and failure to ensure drugs? identity, strength, quality, and purity.

Political leaders need to consider the impact of the biopharmaceutical industry on the economy.
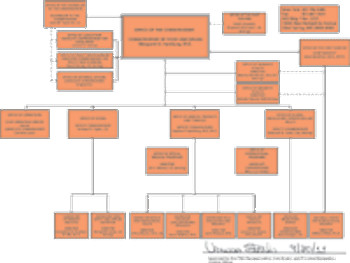
Added responsibilities and outside concerns prompt overhaul of agency's structure.

Readers react to the economic turmoil of the past year and look longingly forward to 2012.

Clamor mounts over compromised care and rising costs due to lack of crucial therapies.
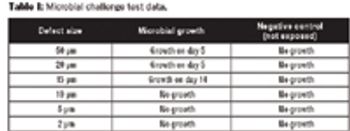
Defects as small as 10 μm can be detected without compromising product cleanliness using helium integrity testing.

In this quarter's column, highlights from the IBC Single-use Applications meeting, the PDA Single-use Workshop, and the BioProcess systems Alliance International Single-Use Summit are presented.

Single-use systems continue to gain traction among biomanufacturers, especially CMOs.
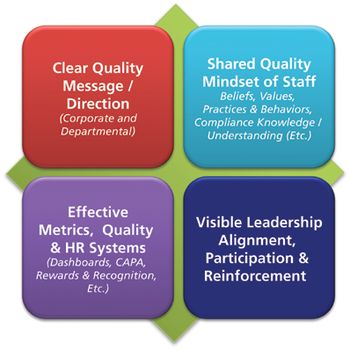
In a culture of quality, it is important that employees adopt this mindset, not because they have to, but because they understand the importance.
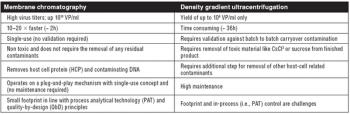
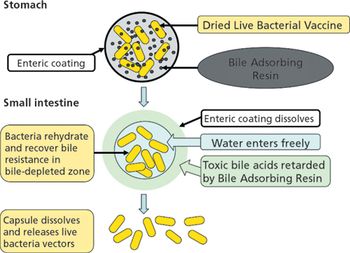
An evaluation of the technologies needed to develop a safe, effective, and economically efficient vaccine. This article is part of a special section on vaccines.
Development of the ideal DNA vaccine requires the optimization of delivery strategies and plasmid vectors.
An in-depth characterization of maize-derived trypsin revealed an unusual nonconsensus N-linked glycosylation.

Manufacturers fund research and reduce prices to tackle diseases around the world.

Inside the National Institutes of Health

Developing a quality agreement template for single-use systems.

Market considerations and new technologies must be recognized to achieve the full benefits of manufacturing prefilled syringes.
An interview with Oskar Gold, vice-president, key account management and corporate marketing, at Vetter.

The lower price of biosimilars will increase patient access to medicines and spur innovation.
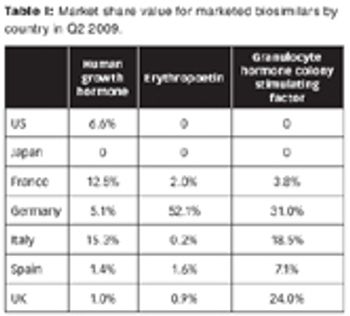
The market landscape for biosimilars is in flux, with limited penetration now, but with the potential for growth for those who can navigate the market. Plus: A SWOT analysis of biosimilars by Anjan Selz.

PDUFA renewal legislation sets stage for new policies affecting revenue, research, and oversight.

Why SOPs are rarely followed, often cited, and in great need of follow-through.

A report commissioned by FDA evaluates the QbD paradigm.
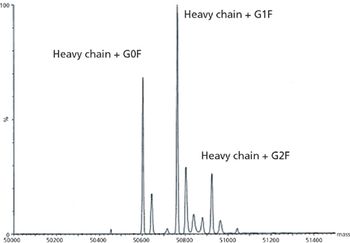
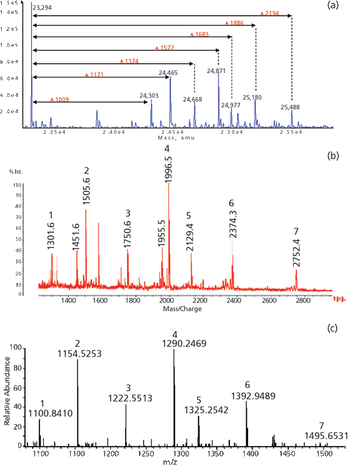
The author describes techniques that can be used to provide the analytical data required by ICH Q6B for characterization of monoclonal antibodies.