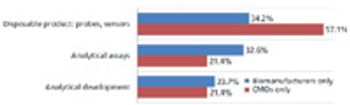
Biosimilar manufacturers need better expression systems and analytical tools to compete.

Biosimilar manufacturers need better expression systems and analytical tools to compete.

Industrializing design, development, and manufacturing of therapeutic proteins.

With the rise in therapeutics comes more complex partnerships.

Single-use systems continue to gain traction among biomanufacturers, especially CMOs.
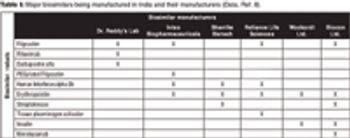
The author explains the current status of India, the challenges, and recommendations that may alleviate these challenges.

A Q&A with Rick Hancock, president of Althea Technologies. This article contains bonus online material.

The BioPlan Associates 8th annual survey identifies key outsourcing concerns of sponsor companies.
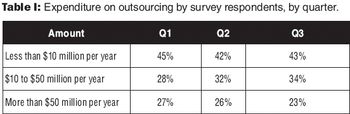
A survey provides insight into drug companies' plans for spending on outsourced services. This article contains bonus online material.
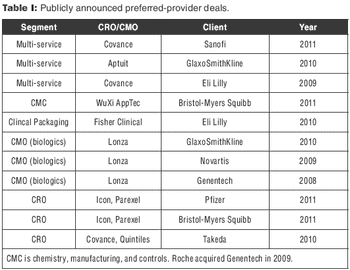
CROs that have made big acquisitions could be outmaneuvered by evolving sourcing models.

Knowing in advance what you need can make a huge difference in a sponsor company's success.

Developing a quality agreement template for single-use systems.
An interview with Oskar Gold, vice-president, key account management and corporate marketing, at Vetter.

The lower price of biosimilars will increase patient access to medicines and spur innovation.

The EU debt crisis portends of possible negative repercussions for the dose CMO industry.

China rises to the top as a destination for international outsourcing.
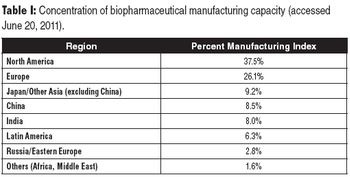
Insights from real-time ranking of global biomanufacturing capabilities.

Incorporating regulatory requirements into the product life cycle is crucial.

Better strategies and practices in sourcing and procurement can contribute to the bottom line.

A recent industry survey shows keen interest in improving bioreactors and cell-culture media.

Knowing where key biomanufacturing facilities are located around the world is essential for decision-making.
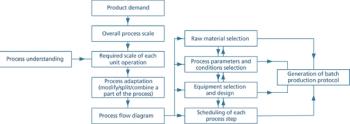
Clear documentation and open communication are essential for effective technology transfer.

Rapid microbial screening provided by contract laboratories can save companies time and money.

Addressing supply-chain challenges.

Mergers and acquisitions expected to increase, as big companies bolster piplines by acquiring smaller biotech companies.

New supply-chain challenges are forcing companies to act in different ways to secure product safety.