
Solutions to circumvent heterogenous mixtures of antibody-drug conjugates and to ensure uniform drug-to-antibody ratio were discussed at a recent Catalent Applied Drug Delivery Institute symposium.

Solutions to circumvent heterogenous mixtures of antibody-drug conjugates and to ensure uniform drug-to-antibody ratio were discussed at a recent Catalent Applied Drug Delivery Institute symposium.

The draft guidance states information concerning a clinical study of a biosimilar should only be included if it is necessary to demonstrate the safety and efficacy of the drug.

Collaboration and single-use technologies aided the rapid scale-up of Ebola vaccine manufacturing

A modular cell-culture platform demonstrates accelerated process development.

Proven science-based strategies can help to accelerate vaccine development.
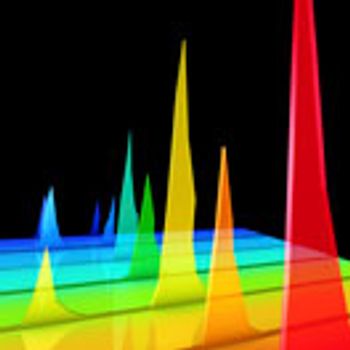
The authors provide application data to support the use of SEC beyond small-scale operations.

Risk management takes center stage, and new mobile apps are being developed to help assess global risks in real time.

Global outbreaks energize vaccine R&D and drive production modernization.

Demand is driving expansion and consolidation of formulation and clinical trial materials services.

Industry experts provide insights on the challenges and importance of using buffers in downstream processing.

Headspace moisture analysis is a rapid non-destructive analytical method that may potentially address the limitations of traditional methods used for residual moisture determination.

Although many immunotherapies focus on tumor-associated antigens, many of the peptide targets on the surface of cells have not be validated.

Adheren relies on chemical modifications, rather than genetic engineering, to create its cell-based immunotherapies.

The companies will provide assays for functional comparability, for use in quality control, lot release, and stability testing of biosimilars and biobetters.

A team of Bristol-Myers Squibb scientists will work in a new laboratory at the National Institute of Bioprocessing Research and Training facility in Dublin, Ireland.

Novo Nordisk broke ground on a facility in Clayton, NC, to manufacture APIs for GLP-1 and insulin medicines.

Collaboration will provide for unified development and manufacture of antibody drug conjugates.

The BARDA-supported monoclonal antibody was approved both as a treatment after anthrax exposure and as an anthrax prophylactic.

The Human Vaccines Project brings together academic research centers, industrial partners, nonprofit organizations, and governments to address the primary scientific barriers to developing new vaccines and immunotherapies.

The agency announced enhanced warnings for immediate-release opioids to inform prescribers and patients of risks related to use.

During a clinical trial of 48 participants, 100% of those that received the TV003 vaccine were protected from infection.

TxCell’s new facilities are based in Sophia Antipolis, France, on the premises of GenBiotech.

This marks the first time a biologic manufactured in China has been released for use in a clinical trial in Europe.

The pharma company revealed in a fourth quarter call that it will improve its cell-culture capabilities by focusing on the use of naïve, highly proliferative cells to manufacture its CAR-T drug candidate.
Asking the right questions is crucial to establishing a biopharmaceutical facility design.