
Microbial models offer some exciting production alternatives.

Microbial models offer some exciting production alternatives.

Miniature bioreactors add value by reducing validation efforts.
The authors provide their perspectives on shipping validation.

Researchers discovered more than 300 antibodies that reacted with the Ebola virus’s surface glycoprotein and could be used to neutralize many strains of the virus.

FDA fast-tracked the monoclonal antibody based on early clinical data from a Phase I trial.

Vaccine R&D has grown exponentially in recent years, spurred by ethical and medical needs to combat lethal infectious outbreaks and increased funding from public and private agencies and organizations.

The agency prepares a plan to implement new packaging safety features.

FDA granted Immunomedics breakthrough therapy designation for the company’s investigational antibody drug conjugate for treatment of triple negative breast cancer.

The International Society for Pharmaceutical Engineering (ISPE) Facility of the Year Awards (FOYA) program announced its 2016 Category Award winners for operational excellence, sustainability, process innovation, project execution, equipment innovation, and facility integration.
The authors review the status of expression of antibodies in microbial hosts and present the recent advances in the production of aglycosylated antibodies in bacteria.
Rapid methods to test CAR-T therapies for potential contamination are on the horizon.

The authors describe the impact of the knocking of the pgi gene of the wild type MG1655 strain on the growth kinetics of plasmid-free and plasmid-bearing cells.
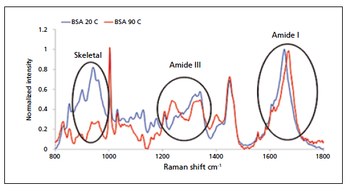
In this article, the author reviews some of the techniques that can yield valuable information on protein stability, focusing specifically on protein aggregation. Emphasis is placed on the enhanced information made available when technologies are used orthogonally, and the alignment of different approaches with specific stages of the biopharmaceutical development workflow.

Novasep is building a new synthesis laboratory and adding capacity for kilogram-scale batches of synthetic molecules that are needed for biological testing and preclinical trials, at its Pennsylvania, US facility.

Serialization, combination products, emerging markets, outsourcing, TVF, and adherence are identified as the key themes in drug delivery and packaging for 2016.

Takeda Pharmaceuticals announced the acquisition of a biopharmaceuticals manufacturing plant in Minnesota.

The Cell Therapy Catapult, University of Birmingham, and Cancer Research Technology collaborate on CAR-T cell immuno-oncology therapy development.

FDA discusses a new program that allows pharmaceutical companies to submit proposals for new manufacturing technology.

BioPharm highlights the monoclonal antibodies that may gain United States regulatory approval in 2016.
In semiconductor manufacturing, for example, a thorough understanding of process variation allows companies to manufacture circuits with billions of transistors at high yields. These variations are translated into a set of design rules, which help ensure that designs will be manufactured successfully and meet safety and other regulatory requirements.

The authors explore the use of precipitation using polyvinyl sulfonic acid and zinc chloride in place of capture chromatography to reduce the cost of goods in the insulin manufacturing process.
mstay/Getty ImagesBiopharmaceutical manufacturing involves a series of complex unit operations linked together to provide high-purity, biologic actives with specifi

Traceability and transparency will remain elusive if manufacturers continue to approach serialization projects on a case-by-case basis.

The results of an industry workgroup’s examination of EMA’s guide on shared facilities are presented.
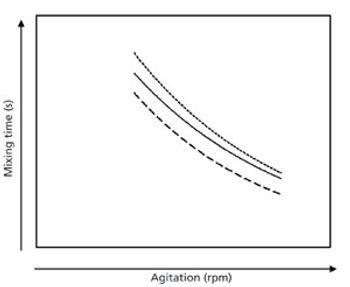
The authors conclude that miniature bioreactors can adequately predict the cell culture kinetics in scaled-up reactors using equal mixing times.