
FDA sets a July 2016 deadline for the final version of the rule on labeling changes for approved drugs and biologics.

FDA sets a July 2016 deadline for the final version of the rule on labeling changes for approved drugs and biologics.

Paragon Bioservices entered into a contract with the International Aids Vaccine Initiative for the process and analytical development and cGMP manufacturing of an HIV vaccine candidate.

Seqirus, CSL Limited’s influenza vaccine business, announced the opening of their corporate headquarters in the United Kingdom.

Biogen, Genentech, Johnson & Johnson, Novartis, and Patheon publicize their support for action on climate change.

Subjective visual evaluation of freeze-dried products can be quantified through mechanical methods of characterizing the properties these materials.

Use of a subspace model is a viable method to characterize process space variables and optimize process performance.

Growing differentiated cells from stem cells may now be a bit easier than before, thanks to the findings from a new study on the production of liver cells. The study, backed by the Centre for Regenerative Medicine and published in Stem Cell Reports, finds that laminins may be a crucial element for the successful clinical-scale production and culture of stem cell therapies. The UK Regenerative Medicine Platform, the European Union Seventh Framework Programme, and the German Federal Ministry of Education and Research funded the research.

The University of Sheffield has appointed Cobra Biologics to advance novel fusion protein technology into Phase 1 clinical trials.

There’s renewed optimism in the biomedical research community that years of effort finally may begin to pay off for developing cutting-edge gene and cellular treatments for debilitating and life-threatening conditions. Jill Wechsler reports.
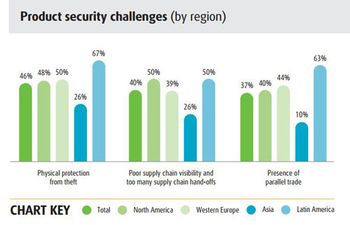
The 2015 UPS supply chain survey suggests that pharma companies need to improve cost control and planning for unexpected events.

Baxter expands capacity for lyophilized cytotoxic oncology therapies at its fill/finish facility in Halle, Germany.

Baxter expands capacity for lyophilized cytotoxic oncology therapies at its fill/finish facility in Halle, Germany.

Panelists at the meeting will focus on clinical trial design, immunogenicity, and enhancing implementation plans for administering already-licensed vaccines to this patient population.

Lawsuit alleges birth control packaging error led to 113 unwanted pregnancies.

The UK’s National Biologics Manufacturing Centre will use Novasep’s BioSC Lab for protein purification.

GE Healthcare's KUBio prebuilt modules were shipped from Germany to JHL Biotech in Wuhan, China.

The new IVIG products boast improvements in purity, safety, and yield.

South Africa’s Biovac Institute, which develops and produces vaccines for the country, launched a public-private partnership with Pfizer to enable local manufacturing of Prevenar 13, a vaccine against pneumonia-causing bacteria.

This case study reviews how quality-by-design principles can be implemented in an intermediate chromatography purification step that uses cation-exchange chromatography.Abstract

Two experts discuss best practices to achieve acceptable sterility assurance levels for aseptically filled products.
The global supply chain for bovine and porcine heparin and regulatory considerations are examined.

Virtual pilot programs examine scenarios that may occur while implementing serialization requirements for the US Drug Supply Chain Security Act.
The authors present a review of the techniques commonly used for glycosylation analysis.

The “next-generation” design for the pods will build on Pfizer’s existing modular prototype for oral solid-dose manufacturing.

The novel antibody-engineering platform works differently than most of the currently employed antibody-modifying technologies, according to UM Baltimore.