
The agency gives an update on the regulation of combination medical products.

The agency gives an update on the regulation of combination medical products.

Scottish injectable-drug manufacturer Symbiosis Pharmaceutical Services plans to expand its sterile filling facility.

Avantor released a packaging platform that allows for the direct dispensing of chemicals-such as salts, buffers, and cell culture ingredients-into processing equipment.

Hiroaki Suzuki will lead Vetter’s business activities in the new Tokyo-based office.

Althea is expanding its existing biological drug product manufacturing operations to include highly active materials, such as antibody-drug conjugates, in a new facility near San Diego, CA.

The company presented a portfolio of new products during the meeting in Madrid.

Product labels should include more information on extrapolation, interchangeability, and the original source of clinical trial data, according to a survey of those in charge of dispensing medications.

Cell Therapy Catapult will provide manufacturing scale-up services to enable Asterias’ future clinical trials and commercial supply for Asterias’ allogeneic dendritic cell immunotherapy, AST-VAC2.

The Bothell, WA location will help the company reach its goal of expanding its global capacity by more than 30,000 liters.

The challenge of achieving zero visible defects (i.e., particulates) in parenteral drugs will require a coordinated effort at all stages of the supply chain, particularly in the production and filling of primary containers.

The Cell History File is designed as a “cell passport” for developers and manufacturers of tissue- and cell-based medicinal products.

Probiodrug has signed an agreement with Rentschler Biotechnologie GmbH for the development of PBD-C06, a pGlu-Abeta-specific monoclonal antibody, as treatment for patients with Alzheimer’s disease.

The agency provides recommendations for submitting proposed labeling with abbreviated new drug applications.

BioPharm International eBooksVolume 28, Issue 14 Single-use technologies including polymer film bags, tubing, filter capsules, and appropriate connectors have become widely accepted in bioprocessing of proteins and vaccines.

Isolators offer a safety and economic advantage for next-generation regenerative medicine products.

Rafe Swan/Getty Images; Dan WardIrreproducible preclinical research is a global, expensive, and well-recognized problem that contributes to delays a
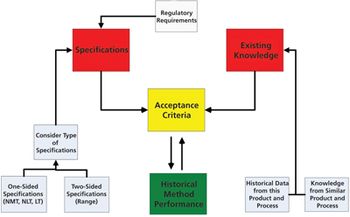
The authors present the results of a survey of biologics manufacturers to evaluate how these manufacturers transfer analytical methods.
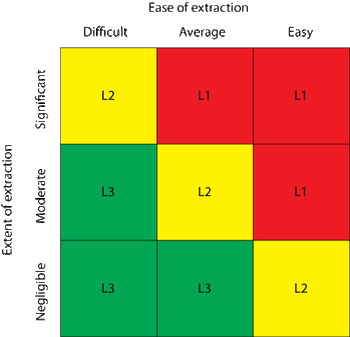
The author explores a dual-supplier sourcing strategy for single-use products and its ability to mitigate business continuity risk.

Vetter plans to invest approximately 300 million Euros during the course of five years to expand drug product manufacturing and logistic services in Germany; upgrades will include an improved RABS system for aseptic processing.

The National Biologics Manufacturing Centre will provide companies with open access to bioprocessing facilities and expertise to expedite low-risk market entry of complex biologics.

Although the Committee for Medicinal Products for Human Use (CHMP) gave Blincyto a positive opinion, full approval of the drug in the EMA will rely on additional clinical studies.

Novo Nordisk will build a local manufacturing plant for FlexPen prefilled insulin delivery devices in Iran.

The fully humanized monoclonal antibody is licensed to Bristol-Myers Squibb.

Under terms of the agreement, Amgen will license Xencor’s XmAb technology platform for five Amgen programs and one Xencor program.