
FDA announced the recall, citing deficiencies in Medistat’s aseptic processing areas and in its environmental monitoring procedures.

FDA announced the recall, citing deficiencies in Medistat’s aseptic processing areas and in its environmental monitoring procedures.

Aragen Bioscience has licensed ProteoNic Biotechnology’s 2G UNic recombinant protein production technology, which increases manufacturing efficiency and reduces cost of goods for recombinant biologicals.

FDA warns that compounded or repackaged drugs stored in certain syringes made by Becton-Dickinson may lose potency because of an interaction with the rubber stopper.

Reuters reports that Novo Nordisk will invest 500 million Danish Krone (approximately $75 million US dollars) into a new 19,000 m2 warehouse in Hillerød, Denmark. The warehouse will handle all inbound raw materials for Novo Nordisk's production in Denmark and will have a capacity of around 17,000 pallets of product, according to the news outlet.

China’s emergence as a significant commercial market is forcing manufacturers to re-evaluate their overall business model.

Three case studies illustrate some analytical methods important for stability testing.

Under the terms of the agreement, Sartorius Stedim Biotech will manufacture membrane adsorber technologies for GE, which will be marketed as part of GE’s ReadyToProcess product offerings.

UPS joins the Global Health Supply Chain Technical Assistance program to help secure the drug supply chain.
Focusing on niche and specialty service offerings gives contract biomanufacturing organizations an opportunity to differentiate in a crowded market.

Sorendls/Getty ImagesTo maintain a state of control and comply with regulatory authorities, many pharmaceutical, biotech, and medical-device companies have adopted continued pro

Adents’ Pharma Suite serialization software features track-and-trace capabilities.
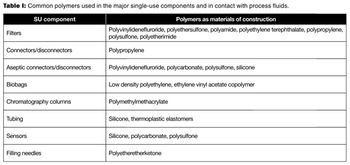
The author discusses the current best practices in technical qualification of single-use systems.
New single-use technologies and other filtration systems are beginning to address cost, throughput, and manufacturing footprint demands.

The Pre-Connect Congress will explore pharma industry trends, such as mergers and acquisitions, the biologics market outlook, and innovation in drug delivery among others.


The TRUST project will focus on the process development of Col-Treg, which is TxCell’s autologous collagen type II specific Treg immunotherapy product for the treatment of autoimmune uveitis.

Shire requests a technical transfer from Sanquin to widen its manufacturer base for the production of Cinryze.

Biopharmaceutical company BeiGene plans to build facility in Suzhou to expand its clinical capability and prepare for commercial manufacturing.

Licenses could potentially bring antibody R&D to areas beyond oncology, such as diabetes treatments

WuXi said its biologics manufacturing business will be one of the company’s key growth drivers over the next several years.

Juno Therapeutics shared some of its business strategies related to process development and manufacturing in a second-quarter earnings call on Aug. 12, 2015. According to CEO Hans Bishop, the company believes its progress in the manufacture of chimeric antigen receptor (CAR or CAR-T) T-cell products will drive the company’s success for all of its product candidates and will be especially important for JCAR015, its investigational treatment for acute lymphoblastic leukemia (ALL). FDA cleared the investigational new drug application for JCAR015 on July 30, 2015.

The partnership will help divert 840 tons of waste related to single-use products from landfills or incineration during the next year alone.

CMC Biologics will manufacture monoclonal antibodies (mAbs) and provide process development services for the PATH Malaria Vaccine Initiative.

The company closed its Zebulon-based plant after routine testing of a cooling tower revealed the presence of the bacterium responsible for Legionnaire’s disease.

GEA's self-contained homogenizer is designed for laboratory applications, including cell dispersions.