Antibody fragments pose unique challenges in recovery, purification, and formulation.

Antibody fragments pose unique challenges in recovery, purification, and formulation.

NIBRT's Ian Nelligan on what to expect when starting a downstream process.
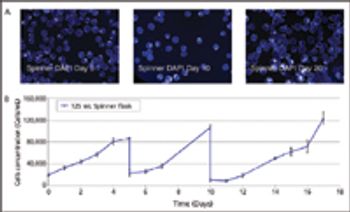
Demonstration of large-scale stem-cell scale-up.

This month, we rewind to "Separations Technology Outlook, Part II: Improved Recovery and Greater Purity."
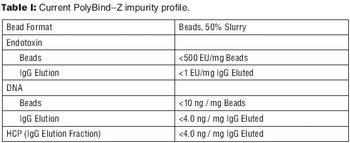
The authors discuss an alternative to traditional Protein A resins.
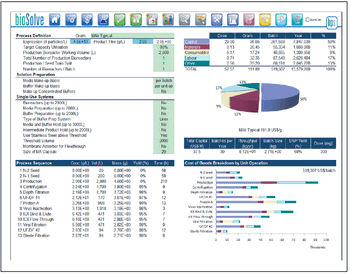
An analysis of flow rate, load density, viral clearance, and cost.
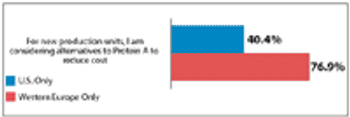
Biomanufacturers and vendors are now exploring more cost-effective purification technologies.

Membrane chromatography ensures purity at high flow rates.
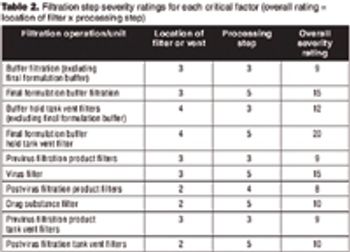
Conducting a FMEA analysis is a good first step in a risk-based approach for determining the need for a filter integrity test.
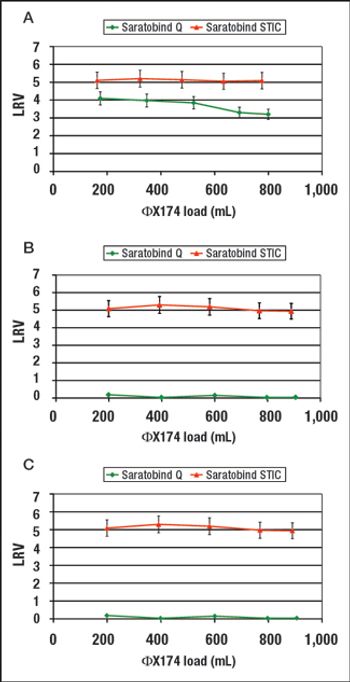
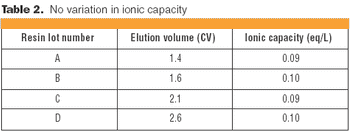
STIC allows polishing to be carried out without an interstitial dilution step, which reduces process time and avoids additional buffer preparation and hold steps.
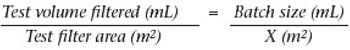
Why staining is crucial in flow decay studies.

A purification scheme to maximize the efficiency of the purification process and product purity while minimizing the development time for early-phase therapeutic antibodies.
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.
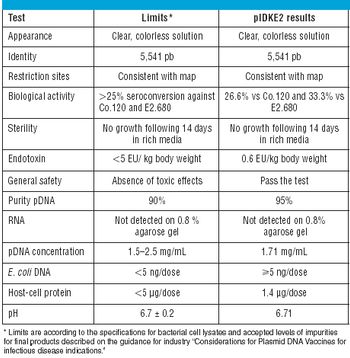
The Center for Molecular Immunology (Havana, Cuba) has been working on a novel cancer immunotherapy targeting the epidermal growth factor (EGF). The vaccine is composed of a chemical conjugate of EGF and a carrier protein (rP64k), designed to trigger an anti-EGF antibody response. The results of studies of molecular characterization, immunogenic activity, and clinical data are presented here.

An analysis of current and upcoming industry challenges.
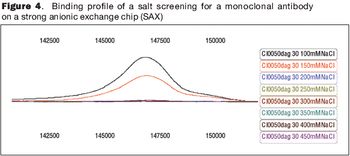
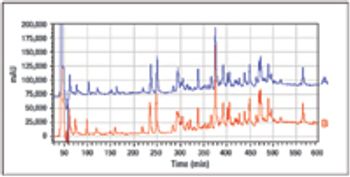
Understanding the impact on process performance.
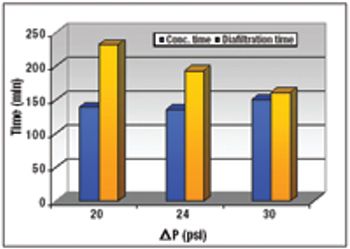
An alternative approach to traditional Protein A schemes is comparable in overall efficiency, product recovery, and quality.
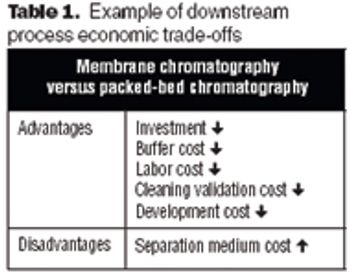
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.

When platform processes are applied to fusion molecules, innovation and flexibility are needed.

Low-pressure process chromatography could not have developed without immense efforts to resolve scale-up issues in both column design and matrix stability.

Membrane-based chromatography technologies sometimes offer advantages over resin-based technologies.
To shorten time to market for new therapeutic proteins, new and fast methods, such as high throughput screening, are needed to speed up downstream processing. The platform technology discussed in this article includes a structural approach that can be used as a general procedure to purify therapeutic proteins. The approach starts with ligand screening and selection-on-a-chip, with the Surface Enhanced Laser Desorption Ionization–Time of Flight (SELDI–TOF) mass spectrometer system. Next, resin screening and supplier selection are performed using robotics, followed by scouting studies under dynamic conditions to select the best resin. Finally, optimization studies of critical parameters are carried out with statistical design approaches (design of experiments). A few examples are presented to explain the platform approach for purification development in more detail.
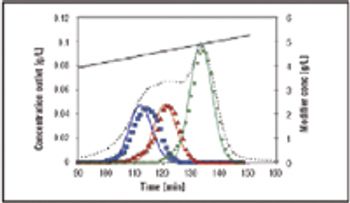
This article presents the multicolumn countercurrent solvent gradient purification (MCSGP) process, which uses three chromatographic columns, and incorporates the principle of countercurrent operation and the possibility of using solvent gradients. A MCSGP prototype has been built using commercial chromatographic equipment. The application of this prototype for purifying a MAb from a clarified cell culture supernatant using only a commercial, preparative cation exchange resin shows that the MCSGP process can result in purities and yields comparable to those of purification using Protein A.
Biopharmaceutical processes typically require a significant investment in equipment-often a substantial obstacle for start-up companies. The risk of drug development failure is often high, further limiting access to the required capital. Flexibility and lower capital outlays are required not only by start-up companies, but also by research organizations with multiple product lines and by companies requiring quick capacity increases. Disposable technologies offer the highest potential for these companies to meet their business requirements. With lower capital requirements and increased flexibility, disposables are an important part of these companies' risk management strategy.