
Viral clearance processes and guidance must evolve along with newer biotherapeutic modalities.

Viral clearance processes and guidance must evolve along with newer biotherapeutic modalities.

Continuous SEC was shown to increase productivity with the same product quality and yield.

The 6th-generation Eclipse FFF system handles analytes such as proteins, polymers, viruses, gene vectors, and liposomal drug nanoparticles.
A balance must be achieved between debris and impurity removal and maximizing product quality and yield.
As technology matures, inefficiencies and process limitations in downstream process chromatography are improved.
New therapeutics modalities and the need for greater process efficiency are driving technology development.
Developers need to transcend the limits of existing separation technologies, to maximize vector recovery while preserving therapeutic potency.
The authors of this study demonstrate an innovative method that is useful and complements traditional HA assays.

The companies will focus on identifying peptides applicable to the affinity chromatography process used in the purification of biopharmaceuticals.

The new program, Cornerstone, integrates process development expertise and novel technology to remove development bottlenecks in the manufacture of gene therapy medicinal products.
Higher cell densities are driving innovations in harvesting, including closed systems for intensified processes.

Single-use technology is gaining ground in downstream bioprocessing, but challenges stall further adoption.

The company’s Quantum peristaltic pump uses a patented single-use cartridge technology and is applicable to downstream bioprocessing.

Pharmaceutical Technology and BioPharm International will present a Keynote Session on Meeting Bioprocessing Manufacturing Capacity Demands on Wednesday, April 3, 2019, during INTERPHEX 2019 at the Javits Center in New York City.

Laboratory test methods evaluate cleaning agents and cleaning process design for removing resin residues from the surfaces of non-dedicated chromatography columns systems.

Downstream process equipment for mAbs manufacturing must be designed to fit technology developments in upstream processes.
New ligands are being developed to meet the separation and purification needs of next-gen biologics.

A key technology that can help achieve a continuous production flow is single-pass tangential flow filtration.
This research proposes a method for separating and purifying tissue-type plasminogen activator from a fungal cell source.

The investment builds on a collaboration the companies entered into in 2007 for various biomanufacturing projects.
The second half of this article describes the testing methods used by the authors to demonstrate the applicability of single-use mixing technology for virus inactivation.
This article explores the use of single-use mixing technology in a detergent-based virus inactivation step during a monoclonal antibody production process.
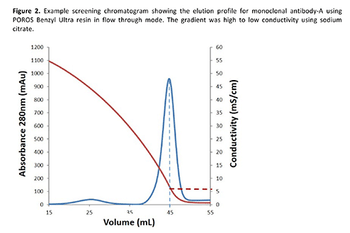
Hydrophobic interaction chromatography (HIC) in flow-through mode offers a more efficient and cost-effective polishing/purification process to remove monoclonal antibody aggregates while maintaining purity at ≥99% than a mixed-mode bind/elute procedure.
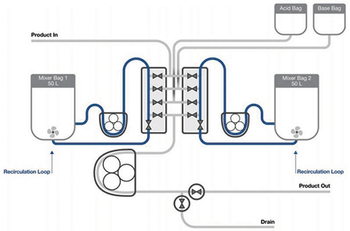
Testing demonstrates an automated semi-continuous process strategy for viral inactivation with steps that mimic batch processing.

The new features on the purifier deliver additional sizes and sterilization/sanitization compatibility.