
The year 2007 witnessed the approval of fifteen biopharmaceuticals in the United States and European Union.

If Indian biologics manufacturers can establish a track record for recombinant products, enhance quality image, maintain cost competitiveness, and demonstrate technology transfer and regulatory knowhow, they are likely to be in the middle of the next boom in biologics manufacturing.

The year 2007 witnessed the approval of fifteen biopharmaceuticals in the United States and European Union.

An analysis of current and upcoming industry challenges.

To achieve the right balance between disposable and reuseable options, companies must consider important technical and economic factors.
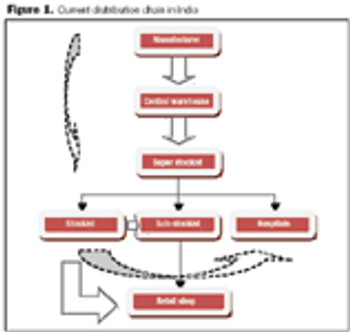
The rapid growth of the pharmaceutical industry in India is yet to create significant changes in the Indian distribution system.

The biotech industry's year-to-date report card contains good grades despite the turbulent economic climate.

There are several challenges associated with protecting patents for personalized medicines.

Every biotech company reaches a point in its development where it must decide what path it will take after it passes the start-up phase. This article discusses what the company must consider to decide what business model it will follow.

Risk mitigation should be a key aspect of any contract manufacturing organization's business strategy.

Manufacturers of biopharmaceuticals can improve productivity by taking patient wellness into account.

What the Indian government is doing to make its biotech sector as strong as its IT sector.
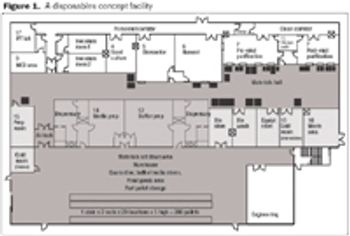
In addition to making technical developments, vendors are also looking at ways to improve supply-chain security. By offering standard, off-the-shelf products, vendors are able to shorten lead times and improve the security of supply.

The US Food and Drugs Administration is boosting its efforts for orphan drugs development.

Best practices a pharmaceutical company can follow to execute their strategy sucessfully.

The bioinformatics industry is currently one of the fastest growing fields in India's biotechnology sector. Indian IT companies have several advantages in the bioinformation field and can continue to grow their opportunities worldwide.
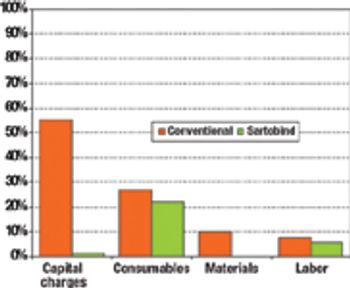
The current focus on cost-of-goods (COGS) models is underplaying the benefits of disposables technology in biopharmaceutical manufacturing. The best method for accounting for the benefits of reduced and delayed capital expenditures is through the use of NPV analysis.

It is important to understand critical aspects of the CMO's capabilities. Only by auditing certain key areas can the sponsor be assured of the quality of the materials produced.

The comparative research approach may be preferable to price controls in the guise of government negotiations for the Medicare drug benefit, coverage denials, and limits on access to new technologies.
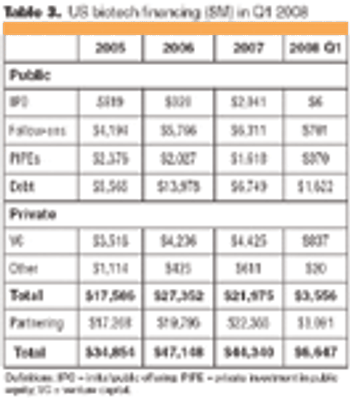
Following the market crisis of the first quarter of 2008, biotech IPOs and financing are down, but partnering continues, and mergers and acquisitions (M&As) remain hot.
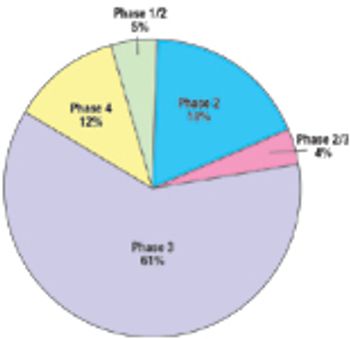
Most local and global clinical research organizations (CROs) consider an operational presence in India as key to their overall business plans. India is clearly on course to become the next hub for clinical trials.
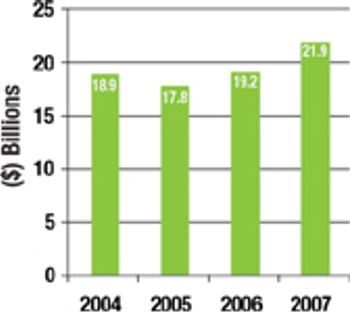
The current overcapacity situation in the bio/pharmaceutical industry is a reminder that CMOs need to come up with business models and value propositions that are based on more than just selling capacity.

Recent problems with food and pharmaceutical ingredients sourced from China highlight a major disadvantage of our complex international supply chains for food and drug ingredients. A global supply chain offers more opportunities for accidental contamination as well as intentional adulteration and counterfeiting. Sticking to minimal requirements may not be enough.
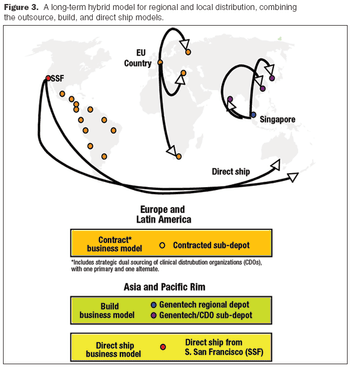
A decision-criteria matrix and cost models helped pinpoint the best distribution approach for the short- and long-term.
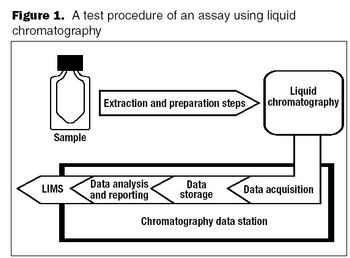
The outsourced service provider should be considered an extension of your own laboratory.

After a strategic evaluation of what activities to outsource, sponsor companies should follow key guidelines for selecting and auditing a provider and preparing quality agreements.
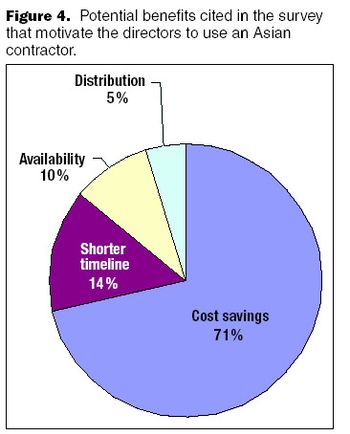
When a biopharmaceutical company pursues an outsourcing strategy, the choice of a contractor is a critical and strategic decision.