
Use Lean techniques to improve manufacturing compliance

Use Lean techniques to improve manufacturing compliance

How to optimize facility utilization and improve plant performance.

Several legal considerations are key to protect the buyer and seller in royalty interest transactions.

Avoiding healthcare reform is not the best option for the pharmaceutical industry.

It is essential to build and maintain a good working relationship between the client and contract research organization.

The current competitive environment is forcing service providers to evaluate their business models and focus on value and performance.

Big biotechs will do just fine in the ongoing financial crisis, but the smaller companies will have more difficulty weathering the storm.

Biodefense start-up companies have an abundance of options when seeking funding.
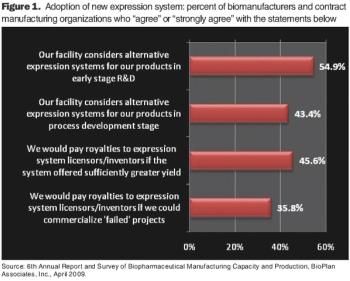
With all of the new expressions systems being developed, companies must decide what improved production and yield are really worth.

The biopharmaceutical industry must continuously evolve to keep up with changing trends.

At some point, will heavy investments in large, stainless-steel based facilities become a burden to US companies?

How change plays out will depend not only on the new Whitehouse, but on pharma leaders' ability to adapt to changing times.

What the current lack of venture capital means for the CRO market.
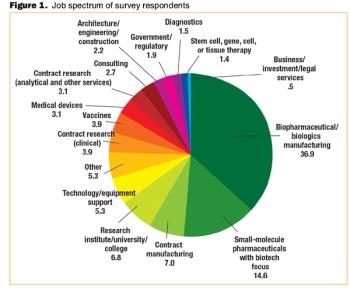
Will the global economic crisis affect your job? BioPharm International's third annual salary survey finds out.

Small biotechs were forced to restructure and downsize.

Looking ahead at the biopharmaceutical industry into 2009.

The development of a skilled labor force is essential for an expanding biopharmaceutical industry.
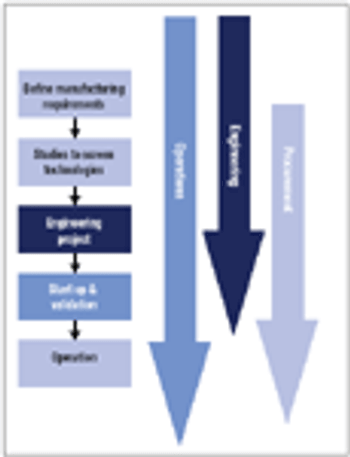
In new disposables projects, it is critical that engineering, procurement, and operations groups work together early on to manage supply chain risk.
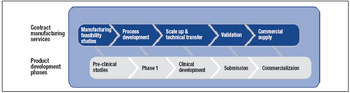
Four reasons why outsourcing may be the best option, and key factors to consider when selecting a provider.

To stave off quality issues associated with production processes, a centralized and enterprise-wide quality management initiative must be enforced.

No time for QbD? How to convince management to make it a priority.

A closer look at the past year's development in India.

The meltdown in the financial markets represents a sea change in the world of financing that will continue to affect the flow of much needed capital into the sector for the foreseeable future.

There could be a serious glut of commercial scale mammalian cell culture capacity over the next five years. Then again, there could be a significant shortage. It all depends on how things develop in expression technology, the new product pipeline, and corporate strategies.
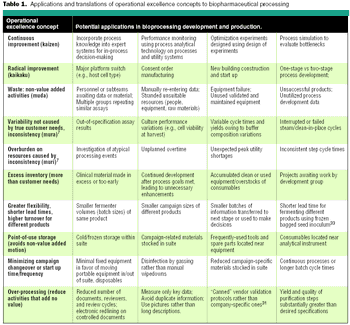
Translate the concepts in to practical applications and reduce waste.