
Biotech's mid-sized elites position themselves for growth.

Biotech's mid-sized elites position themselves for growth.

An in-depth analysis of the patent provisions of the new legislation.

In light of the new ruling, patent licensees may want to re-evaluate the strength of their licensed patents.

After a bright start to the year, some of biotech's blue chip companies have seen their early gains turn into losses.

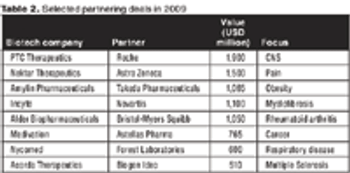
Thomson Reuters' Mark Gordon looks at how real partnership opportunities are developing in a virtual pharma world.
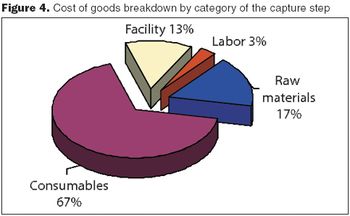
Combine cost analyses with QbD to improve operations and lower costs.

The industry must be prepared for the impact of the sweeping changes expected from the new legislation.

As the capital markets in the US and globally continue to strengthen, the biotech industry can expect to see a rise in initial public offerings this year.

This year's BIO International Convention will be occurring against a backdrop of profound change in our industry.
The companies that have survived the financial meltdown are well placed to adapt to the new environment that we now are entering.
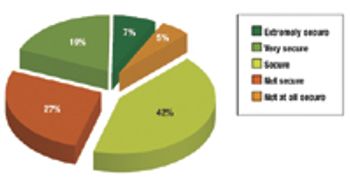
The year 2009 was marked by recession, industry megamergers, and venture-capital scarcity. How did biopharmaceutical professionals fare?

Gaining a license can be a complex process, but a few key tips can help you avoid common pitfalls and patent infringements.
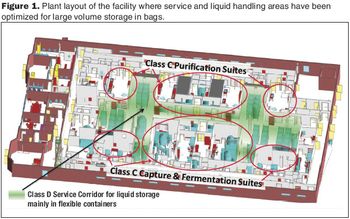
A case study compares capital costs, operating expenses, and NPV for a new MAb plant.

Drug counterfeiting has become a major problem for the FDA today; a variety of solutions is needed.
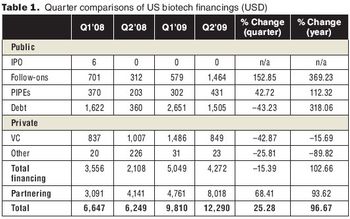
Biotech impressed investors with positive drug data, strong drug sales and earnings, and partnering deals.

Ten biopharmaceuticals gained marketing authorization in the US or EU in 2008, although only four were new approvals.
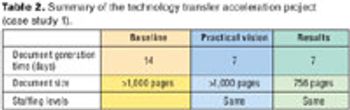
A step-by-step approach is essential for successful implementation.

Small changes can have a big effect further downstream in your manufacturing processes.

To assess current trends in outsourcing, BioPharm International turned to Mark Douglas, PhD, strategic business development manager, Avecia Biologics Ltd; Geoffrey M. Glass, senior vice president of strategy, corporate development and business integration, Patheon Inc.; Rob Gustines, director of marketing, KBI Biopharma, Inc.; Jon S. Kauffman, PhD, director, method development & method validation and biopharmaceutical services, Lancaster Laboratories; Roger Lias, PhD, president, Eden Biodesign, Inc.; John A. McCarty, vice president, formulation sciences and drug delivery, Azopharma; and Michiel E. Ultee, PhD, vice president, process sciences, Laureate Pharma, Inc.

To assess current trends in outsourcing, BioPharm International turned to Mark Douglas, PhD, strategic business development manager, Avecia Biologics Ltd; Geoffrey M. Glass, senior vice president of strategy, corporate development and business integration, Patheon Inc.; Rob Gustines, director of marketing, KBI Biopharma, Inc.; Jon S. Kauffman, PhD, director, method development & method validation and biopharmaceutical services, Lancaster Laboratories; Roger Lias, PhD, president, Eden Biodesign, Inc.; John A. McCarty, vice president, formulation sciences and drug delivery, Azopharma; and Michiel E. Ultee, PhD, vice president, process sciences, Laureate Pharma, Inc.

To assess current trends in separation and purification, BioPharm International turned to Mandar Dixit, head of product management?filtration technologies, Sartorius Stedim Biotech; Günter Jagschies, senior director of strategic customer relations, life sciences, biotechnologies, GE Healthcare; Richard Pearce, program director of purification, Millipore Corporation; and Jon Petrone, global technical director, Pall Life Sciences.

To assess current trends in outsourcing, BioPharm International turned to Mark Douglas, PhD, strategic business development manager, Avecia Biologics Ltd; Geoffrey M. Glass, senior vice president of strategy, corporate development and business integration, Patheon Inc.; Rob Gustines, director of marketing, KBI Biopharma, Inc.; Jon S. Kauffman, PhD, director, method development & method validation and biopharmaceutical services, Lancaster Laboratories; Roger Lias, PhD, president, Eden Biodesign, Inc.; John A. McCarty, vice president, formulation sciences and drug delivery, Azopharma; and Michiel E. Ultee, PhD, vice president, process sciences, Laureate Pharma, Inc.

To assess current trends in information technology, automation, and process control, BioPharm International turned to Rick E. Cooley, market development manager?process analytics, Dionex Corporation, and James Erickson, president and chief executive officer, Blue Mountain Quality Resources, Inc.

Recent patent rulings raise significant patentability questions for DNA sequence inventions.
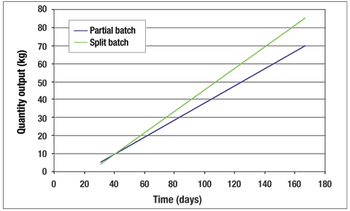
A case study in capturing indirect costs and benefits.