
The contract provider needs to know as much as the NDA holder.

The contract provider needs to know as much as the NDA holder.

Mike Clayman, CEO of Flexion Therapeutics, talks about his company's strategy to focus on a single therapeutic area.

A review of key industry shifts and promises for the future.

Formulators and developers are at the heart of the industry's basic premise-they are saving lives.

Industry experts discuss significant achievements. Plus: What's in store for the future.

Service providers must focus on delivering a superior customer experience.

This month, we rewind to an article titled "The Biotechnology Industry: A First Quarter Snapshot."

Future sponsor-contract provider relationships will require more integration.
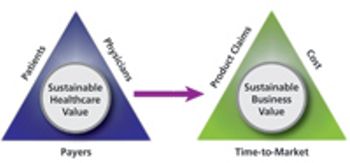
BioPharm talks with Tarja Mottram, CEO of Action for Results, on design-for-value concepts, management, and cross-functionality.

Better news about the global economy buoys life-sciences funding.

FDA has created a dedicated cadre of foreign drug investigators and established permanent offices worldwide.

Rather than seeking a single indication for one large group, the orphan-drug approach segments the market for a drug more minutely.

Soaring opioid use creates challenges for new drug development and supply-chain control.

A closer look at elastomer changeout times provides one example of using industry knowledge to improve operations and cost.
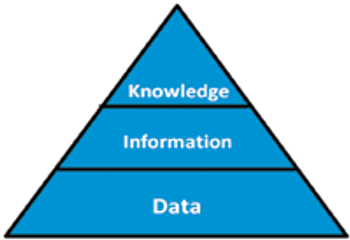
The authors discuss the technology and guidance required to achieve good KM in a biopharmaceutical company.

Recovery audits and other best practices in procurement can improve the bottom line.

BioPharm talks with Tarja Mottram, CEO of Action for Results, about key business considerations when starting out.

Collaboration can begin with a conversation.

The confluence of science, technology, and regulation will provide our industry with the guidance to move forward.

Representatives from leading CROs weigh in on key challenges tied to the biopharmaceutical R&D, including issues regarding bioequivalence, platform technologies, process analytics, and more.
An architectural wonder teaches the biopharmaceutical industry a valuable lesson in project management.

Because of the complexity of the manufacturing processes for biologics, transfer of these processes to a contract manufacturer presents challenges.

Social media use raises questions about applying old standards to new information technology.

Key business considerations when developing biosimilar products virtually.

Has the long-awaited guidance answered all of the industry's questions?