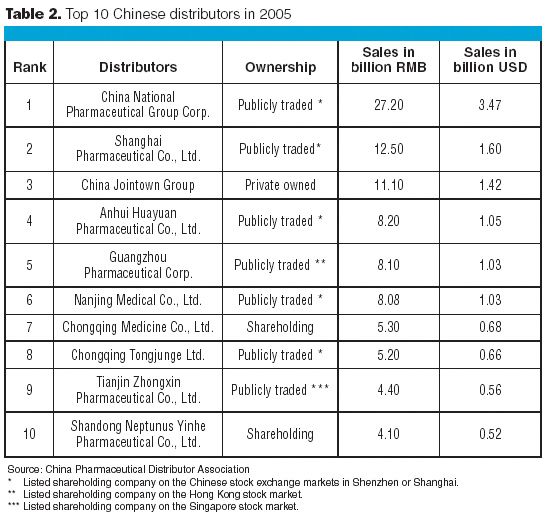
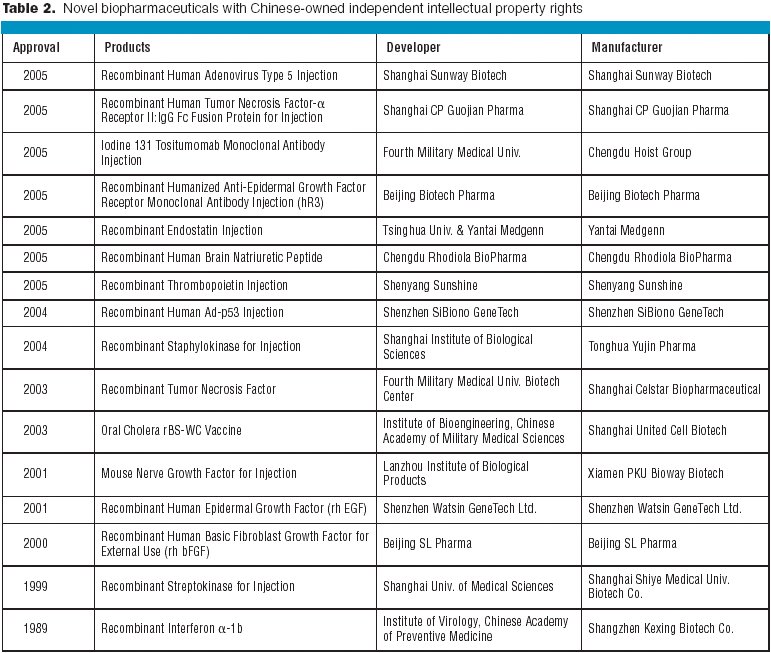
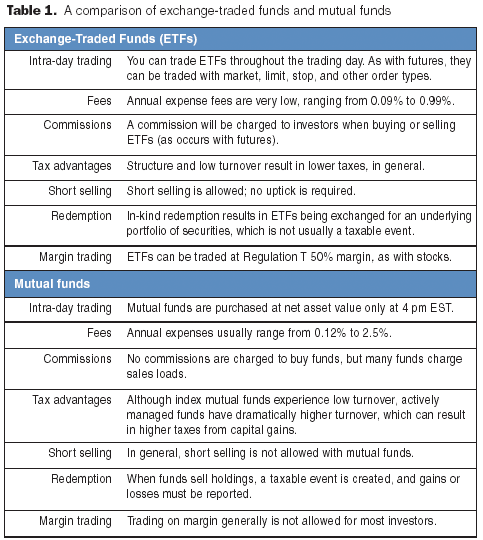
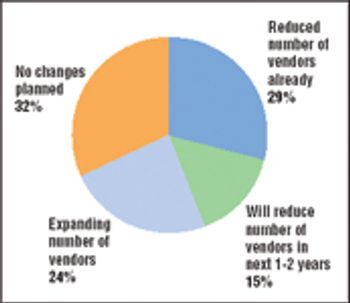
The biotechnology industry is thriving, and hopefully you are too. BioPharm International's first salary and employee satisfaction survey presents a wealth of data that will allow you to compare many aspects of your job with those of your peers. The survey reports on demographics, education, work experience, salary and benefits, and attitudes toward current employment.