The new patent regime is challenging the Indian biopharm industry to transition from generics to novel products.

The new patent regime is challenging the Indian biopharm industry to transition from generics to novel products.

Engaging executive leadership in the quality process is the key to compliance success.

As more biotechs turn to convertible note financing instead of traditional venture capital, they need to be aware of investors' demands.

Did the 2005 Patents Act engender a Western intellectual property rights culture in the country?

The heparin safety crisis puts a spotlight on manufacturing processes and regulatory oversight.

Now is a good time for companies to know their suppliers well.

The supply base for preclinical and clinical development services continues to expand in China.
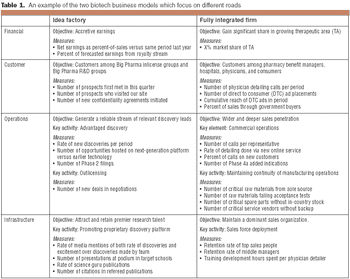
Two business models that companies can follow to map their strategy from concept to implementation.

India is restructuring its regulation of biopharmaceuticals to help the country's industry compete internationally.
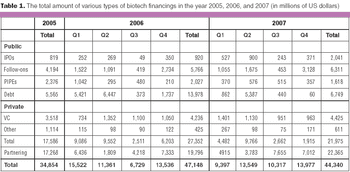
Despite the rising fears about a slowing economy, the biotech industry will continue to maintain its momentum this year.

How an electronics engineer led the first Indian company to carry out indigenous development of a recombinant vaccine.

The cause of the heparin crisis is still unknown. We do know, however, that the FDA is severely underfunded.
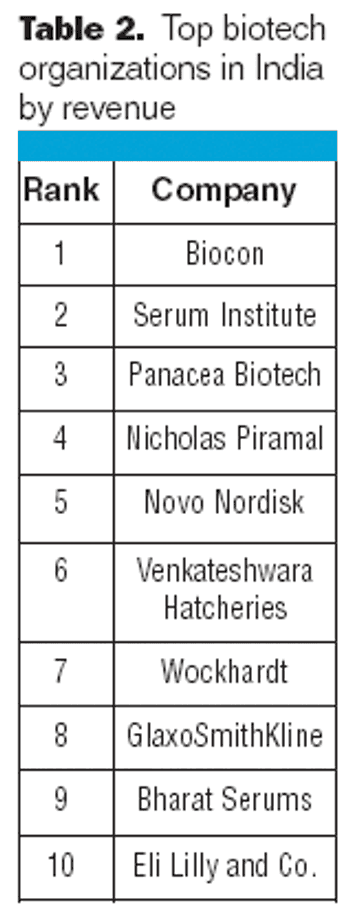
Indian biogenerics could form a major piece of the global biotherapeutics market in the future.

Putting business principles to work in biotechs requires careful implementation of nine critical business systems.

Biotechs can avoid becoming a zombie by having a thorough business plan and a clear focus.

Our second annual salary survey assesses not only how much people earn, but also how they feel about it.

Strategic alliances and partnering deals were a big biotech news story during 2006-with deal values setting an all time record of over $23 billion for the year. The strategic partnering trend continues during 2007 and in this column we present some frequently asked questions and answers about the merger and acquisition (M&A) activity in the life sciences sector.

The Chinese government is making consistent efforts to strengthen IP protection.

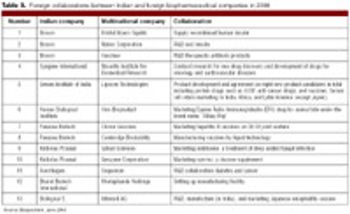
India's biopharmaceutical industry, which was relatively modest only a decade ago, is expected to generate almost $2 billion in sales in 2008, making it one of the largest biopharmaceutical segments in Asia.1 According to BioPlan Associates, Inc., and the Society for Industrial Microbiology's newly published joint study, Advances in Biopharmaceutical Technology in India, the Indian biopharmaceutical industry is growing 25 to 30% per year.1

Eighty drugs will lose patent protection between 2007 and 2011.

Many companies acknowledged that Western regulatory standards are tougher than those in China.

More than $1 out of every $9 under professional management in the United States is involved in socially responsible investing.
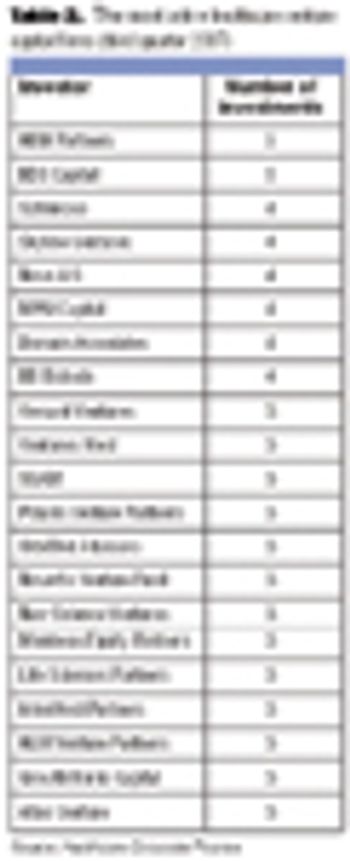
The biopharmaceutical sector can look forward to a financially flush venture funding environment in 2008

The challenge is not in coming up with a list of activities to discard, but in finding a feasible way to stop doing them.
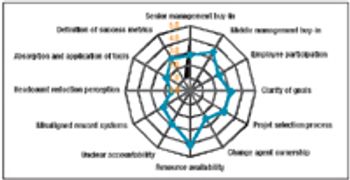
A true visionary leadership is required to drive the progress of operational excellence programs in biopharmaceutical organizations