
The biopharmaceutical industry must consider new ways to manage innovation, including how product data are handled.

The biopharmaceutical industry must consider new ways to manage innovation, including how product data are handled.

Caution should be taken when planning to crowdfund a biopharmaceutical startup.

Higher wages and employment rates give biopharm professionals an edge over counterparts in other industries.

Israel's diverse population, high-quality healthcare system, and resilience to global financial stress make it a strong partner for R'D, clinical research, and market growth.

The employment picture brightened a bit, but biopharma employees still seek better compensation.

The rising cost of drug development and the decreasing proportion of drug-naive population in the US and European markets are driving international pharmaceutical companies to consider emerging markets as a location to conduct their clinical trials. Asia stands out among the emerging markets given its double-digit growth rates.

New regulation offers patients in Brazil greater access to experimental drugs.

Today, there is a global adoption of buying and selling used equipment. This article reviews what organizations should look for when considering the purchase of used equipment.

The pharmaceutical industry grows despite conflict in the Middle East.

The authors review the angiopoietin pathway as an alternative for safer and more efficacious anti-angiogenic therapeutics.

Foreign companies zero in on Myanmar with the hope of securing a foothold in its pharmaceutical market.

Early-stage companies are now embraced by buyers of new issues.

Pharma eyes biologics production in Brazil as the government begins to recognize the potential of these drugs.

PDA/FDA regulatory conference promotes a commitment to quality.

The bio-pharmaceutical business outlook in South Korea remains positive

The US Supreme Court's Myriad decision satisfied both patient groups and patent holders

Pharmaceutical companies have stepped up gene therapy development in the wake of the approval of Glybera in 2012.

Industry players brace themselves to face challenges as India's new drug-pricing policy kicks in full gear.

Fourth-generation biologics, with improved delivery and pharmacokinetics, will continue to drive an overall biologics industry that is already worth over $120 billion.
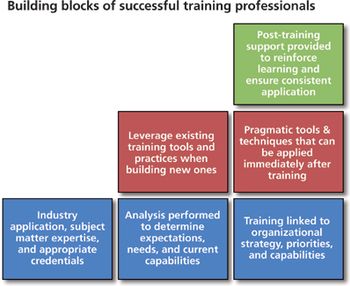
Five key attributes to look for in a biopharm training partner.

Biotech companies raise most money in 13 years.

A unique demographic and payer mix make ASEAN an increasingly attractive region.

Prior to price escalation of pharmaceutical products in Brazil, the country's regulatory authority released a study on price-cap control and its benefits in the past years.

Don Frail, Vice President, AstraZeneca Innovative Medicines Group discusses the company's approach to innovation and the upcoming session at the BIO International Convention

In recent years, large pharmaceutical companies have launched a variety of initiatives to restock ailing pipelines and boost business performance including mergers and acquisitions, diversifying business portfolios to non-pharmaceutical products, downsizing, spinoffs, and entering the biopharmaceutical arena.