
AAPS supports graduate-level programs impacted by cutbacks in funding and resources.

AAPS supports graduate-level programs impacted by cutbacks in funding and resources.

GSK is found guilty of offering bribes to doctors and is fined $489 million.

A dynamic market, industry consolidation, and demand fluctuations lead to a mixed picture of pricing results.

Trends in real estate and facility clusters give insight into the state of the biopharmaceutical industry.

Chinese healthcare reforms may be a double-edged sword for foreign companies.

Data analytic strategies can help companies capitalize on personalized medicine.

New International Council on Biotechnology Associations advocates biotechnology growth.
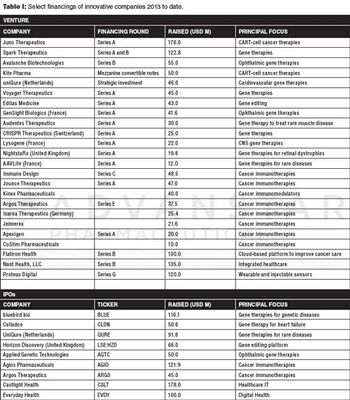
Gene therapy, immune-oncology, and digital healthcare technologies offer investors promise for innovation investments.

Is there potential for growth in Brazil's phytotherapic drug market?

The National Institutes of Health and the National Science Foundation set up biomedical innovation and business program.

Protecting intellectual property rights is vital to biopharmaceutical innovation.

Brunei harnesses its rich biodiversity and the growing halal market in a bid to develop its pharmaceutical sector.

AstraZeneca rejects Pfizer's final offer of $118 billion for a proposed merger of the two companies.

Biopharma companies should not overlook India's growing market.

Sun Pharma acquisition of Ranbaxy Laboratories will create the fifth-largest specialty generics company in the world.

Life-sciences companies continue upward momentum in 2014.

How badly is Brazil's pharmaceutical market suffering from the global instability of emerging markets?
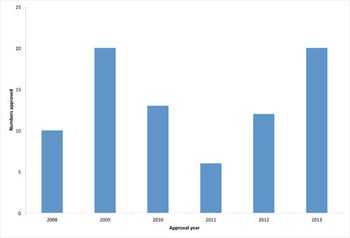
A review of the past year's trends in biopharmaceutical approvals shows an above average approval rate.

China's regulatory and compliance environment is set to change as the government declares a crackdown on bribery scandals.

Conference programming from PDA and BioPharm International expand educational opportunities at Interphex 2014.

Moody's Investor Service reports AbbVie, Amgen, and Roche are the most exposed to biosimilar competition.

Thirteen companies are accepted for participation in the supply chain program.

The biopharmaceutical industry contemplates product innovation within the changing landscape of healthcare.

With numerous biologics set to come off patent soon and the percentage of new therapeutics based on biomolecules growing, demand for contract manufacturing in the biopharma space is heating up.

In 2013, the EMA's Committee for Medicinal Products for Human Use recommended 81 medicines for human use for marketing authorization, compared with 57 in 2012.