Traditional planar culture formats are being superceded by microcarriers for large-scale cell therapy manufacturing.

Traditional planar culture formats are being superceded by microcarriers for large-scale cell therapy manufacturing.
Consider automation early in the rollout of clinical translation and scale up of clinical-trial protocols.

Modeling tools help process engineers optimize a biopharmaceutical facility’s capacity.

FDA noted in a recent inspection that US Stem Cell Clinic was processing and administering stem cell treatments that were neither reviewed nor approved by the agency.

AstraZeneca and Champions will develop new cohorts of patient-derived xenograft models to be used in oncology programs in breast and lung cancer as well as for use in other academic and industry applications.

The first program of this collaboration will focus on the development of a candidate for severe acute pancreatitis.

Pfizer will invest $100 million to expand its manufacturing facilities in Sanford, North Carolina.

Amid debate about “fake news,” peer-review papers offer vital, objective insight.

Media manufacturers are focused on reducing risk, improving quality and consistency, and managing costs.

Managing and prioritizing risk is essential to ensuring raw material quality. USP is developing new guidelines to make the work easier.

ABEC increases the maximum capacity of its Custom Single Run Bioreactors to 4000 L, doubling the industry standard.
The authors present a shift toward more integrated purification processes.

Perfusion processes can attractive for biologics drug manufacturing; however, obstacles remain.

Continuous processing of 100 g of monoclonal antibody in 24 hours has been demonstrated using lab-scale equipment.
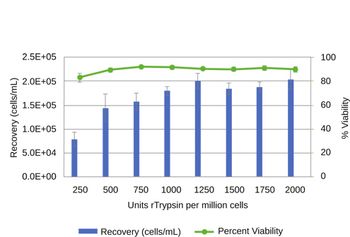
Traditional planar culture formats are being superceded by microcarriers for large-scale cell therapy manufacturing.

Sartorius Stedim Biotech combined the company’s ambr 15 bioreactor system with the Nova BioProfile FLEX2 cell culture analyzer for laboratory experiments.
The success of a truly integrated continuous processing platform relies on the collaborative efforts of upstream and downstream specialists.
This article summarizes the approaches, challenges, and future perspectives for the characterization of N-glycans in biopharmaceutical products.
An increase in biologics raises awareness of particle generation and its role in negative patient outcomes.

BioVectra will open its new microbial fermentation and complex chemistry site, including the capability to handle high-potency APIs, by the end of 2017.

MilliporeSigma released a new surface-active nonionic polymer to ensure lot-to-lot consistency.

Can bioprocessing runs be consistently replicated in an inherently variable production environment?

Improving the bioreactor growth environment increases the rigor of bioprocessing runs.

GE Healthcare continues to ramp up its offerings in the bioprocessing space with the purchase of Asymptote and a continued partnership with Zenith Technologies.
OrlaSURF technology can be used for the development of target-binding assays to monitor the binding of an ADC to its antigen.