
Understanding overall supplier capability versus the critical-to-quality attributes of your product can reduce both risk and cost.

Understanding overall supplier capability versus the critical-to-quality attributes of your product can reduce both risk and cost.

New US Pharmacopeial Convention (USP) standards provide a universal approach to organizing labels for prescription containers dispensed by US pharmacists in an effort to improve patient understanding.

User fees aim to speed approvals and support inspections.

Glenn Thorpe of West Pharmaceutical Services gives an update on packaging and delivery methods for vaccines.
Packaging vaccines using prefilled syringes can increase dosing efficiency, reduce costs, and improve patient safety. This article is part of a special section on vaccines.
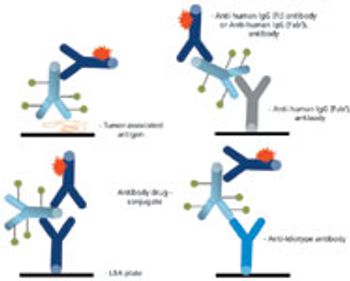
The complex structure of ADCs necessitates different analytical strategies than those for either small molecules or unconjugated monoclonal antibodies.
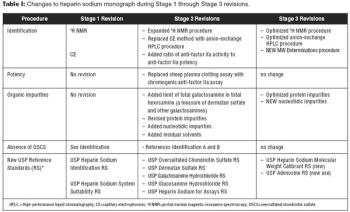
USP optimizes identification tests and impurities procedures.
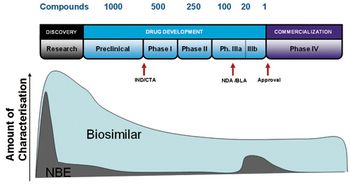
Understanding opportunities and challenges across all major phases of development.

Will the next US President support the backbone of our industry?
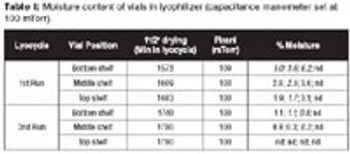
Using an alternate moisture-generation method may provide more accurate data for regulatory submissions.
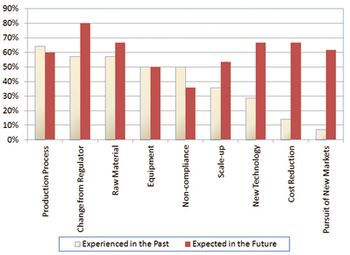
MIT survey results address product and site characteristics that statistically correlate with quality performance.

Anne Marie Dixon of Cleanroom Management Associates, gives us an update on her 1988 article, "Clean Room Management."

The European Medicines Agency (EMA) has abolished its Cell-based Product Working Party (CPWP) and Gene Therapy Working Party (GTWP), with the aim of improving efficiencies and optimising the use of available expertise.

The European Generic Medicines Association (EGA) has raised concerns about the potential fees to be charged by the European Medicines Agency for pharmacovigilance activities

USP Hosts Symposium on Science and Standards

Bristol-Myers Squibb has initiated a voluntary recall of 10 lots of BiCNU (carmustine for injection) previously manufactured by Ben Venue Laboratories, a former, third-party contract manufacturer for the company, to the user level.

The European Medicines Agency has launched a public consultation concerning its inventory of paediatric medicines with the aim of highlighting where further R&D efforts are required. The consultation is the first of its kind in this area.

Using a competency-based approach to effectively train biopharmaceutical industry staff.

The authors demonstrate how an integrated model is helping to achieve regulatory flexibility. This article is part of a special section on biopharmaceutical trends.

PDA's strategic plan calls for maintaining valuable relationships with global regulators.

Brazil's regulatory health authority, Anvisa, plans to establish quality requirements for locally produced pharmaceutical excipients, Anvisa told BioPharm International.

Howard Levine of BioProcess Technology Consultants talks about what industry needs to know to enter the biosimilars game in the US.

Manufacturers and regulators struggle to control phony versions of crucial medicines.

The European Medicines Agency has recommended that the anticancer medicine DepoCyte be recalled from EU countries following the discovery of manufacturing deficiencies at Pacira Pharmaceuticals' San Diego site.

The European Medicines Agency (EMA) will soon be phasing out follow-up measures to marketing authorisations in place of a new system of classification that will be introduced in a stepwise manner.