
ISPE study reveals quality systems of manufacturing as the leading cause of drug shortages.

ISPE study reveals quality systems of manufacturing as the leading cause of drug shortages.

EMA?s revised guideline on biosimilars containing biotechnology-derived proteins is published for public consultation.

USP appoints regulatory experts to elemental impurities implementation advisory group.

FDA cites cGMP violations of finished pharmaceuticals at the company's facilities in Marion North Carolina and Jayuya, Puerto Rico.

FDA Discovers Microbial Contamination in Compound Pharmacy Products

The company is cited for using unapproved visual-inspection methods for finished parenteral drugs and conducting inadequate visual inspections.

Modular containment room at Belfast facility allows studies of biologics and vaccines.

Bills to regulate drug compounding and establish a national track and trace system face political and policy differences.

A science- and risk-based approach to verify and demonstrate that a process operating within predefined specified parameters consistently produces material that meets all its critical quality attributes.

Ranbaxy's $500 million settlement for producing adulterated drugs and fradulent data provides a cautionary tale for patients, FDA, and drug manufacturers.

A unique demographic and payer mix make ASEAN an increasingly attractive region.

Companies can use metrics as a tool to help drive positive change and quality process improvements.

FDA addresses shortages of drugs needed to treat premature infants and patients unable to eat or drink by mouth.

Company receives notice from FDA for not fully investigating foreign particles in APIs and finished products from its facility in Ingelheim am Rhein, Germany.

USP defers implementation date to work closely with ICH Q3D. USP will also form a new advisory group for implementation of the new general chapters on elemental impurities.

The U.S. Pharmacopeial Convention is offering free online access to public standards to help ensure the quality of the herbal ingredients used in medicinal products.

FDA has released guidance on best practices for conducting and reporting pharmacoepidemiologic safety studies.

New Center for Pharmaceutical Advancement and Training increases number of experts and available tools in Sub-Saharan countries.

EMA clarifies biosimilars guidelines.

Manufacturers work with international authorities to harmonize drug registration and supply-chain oversight.

While there are those who want combination products to be controlled by a centralized pharmaceutical-type approval system, the majority of the medical technology industry wants to retain a decentralized device-focused approach.

KR Karu from Sparta Systems spoke with BioPharm International about the importance of having an enterprise quality management system.

The author presents best practices for extractables and leachables.
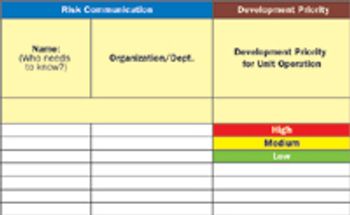
Quality risk management is an essential element of every aspect of drug development and manufacturing throughout the product lifecycle.

FDA issues draft guidance to minimize medication errors.