
Israel's diverse population, high-quality healthcare system, and resilience to global financial stress make it a strong partner for R'D, clinical research, and market growth.

Israel's diverse population, high-quality healthcare system, and resilience to global financial stress make it a strong partner for R'D, clinical research, and market growth.
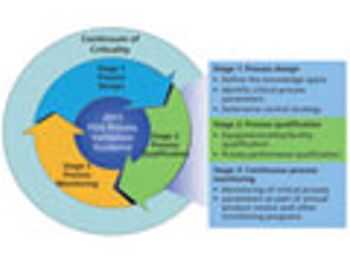
Criticality is used as a risk-based tool to drive control strategies.

The rising cost of drug development and the decreasing proportion of drug-naive population in the US and European markets are driving international pharmaceutical companies to consider emerging markets as a location to conduct their clinical trials. Asia stands out among the emerging markets given its double-digit growth rates.

The international “Fight the Fakes” campaign will raise awareness about the dangers of counterfeit medicines.

European Medicines Agency announces the launch of the Accelerated development of vaccine benefit-risk collaboration in Europe (ADVANCE) project.

USP issues call for candidates for its 2015-2020 Council of Experts.

Biotechnology Industry Organization President James Greenwood writes senators in support of Start-up Jobs and Innovation Act.

FDA releases guidance on pulmonary tuberculosis drugs.

EMA and FDA publish joint QbD guidance on design space verification.
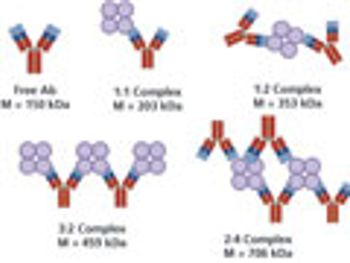
A study to investigate metacomplex formation in the hetero-association of a multivalent antigen, streptavidin (SA), and a bivalent antibody (Ab) using a composition-gradient multiangle light-scattering (CG-MALS) system consisting of a composition-gradient device, a MALS detector, and a UV/Vis absorption detector.

FDA releases a strategic plan and issues a proposed notification rule to improve prevention and resolution of drug shortage problems.
An effective supplier-initiated change management process is discussed.

A new report details FDA's role and responsibilities in personalized medicine.

New regulation offers patients in Brazil greater access to experimental drugs.

Using closed systems opens up many new possibilities for how facilities are designed and operated and may also present lower risk to the operation and, ultimately, the product.

BioPharm International speaks with industry experts about challenges faced in managing the cold chain.

Regulators hope new standards will stop illegal drug imports, but manufacturers fear they may stifle innovation.

Legislators agree on a limited bill affirming FDA authority over compounders while setting up a process for national drug tracking.

USP is developing and revising distribution standards in response to changes in the global supply chain.

The JOBS Act and FDASIA show early signs of accelerating drug development.

The authors discuss how to develop a cost-effective thermally protective packaging system.

Zhoydro ER is the first drug to have updated labeling now required for all ER/LA opioid analgesics.

FDA is seeking a permanent injunction against a dietary supplement manufacturer following the company?s repeated distribution of unapproved drugs and adulterated dietary supplements.

During the ongoing federal government shutdown, FDA activities will be limited to work involving the safety of human life or the protection of property, and activities funded by carryover user fee balances PDUFA, GDUFA, and MDUFA.

The pharmaceutical industry grows despite conflict in the Middle East.