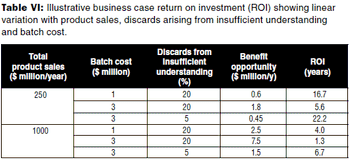
The author describes a methodology for developing a per product qualitative and semi-qualitative business case for applying QbD to a biopharmaceutical product.

The author describes a methodology for developing a per product qualitative and semi-qualitative business case for applying QbD to a biopharmaceutical product.
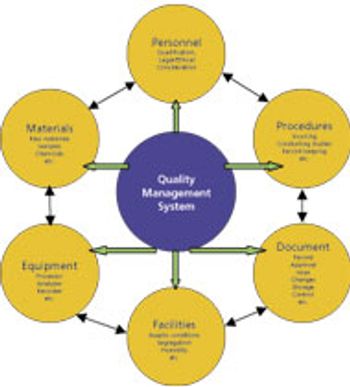
Members from an ASQ working group provide analytical methods to enable PAT.

A report from the European Commission shows that fake pharmaceuticals were the top articles detained by European-Union customs in 2011.

Guide helps companies meet CGMP requirements.

Unnecessary analytical testing can lead to unnecessary costs.

Although FDA cannot do anything to stop drugs from being discontinued, it can do something about supply and quality problems that lead to shortages.

Multiple initiatives are moving forward to maintain US leadership in biopharm R&D.
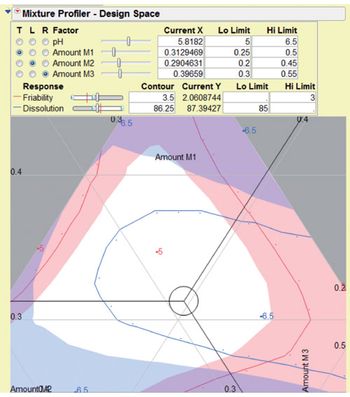
Harmonized regulations call for a risk-based and systematic approach to evaluating and selecting CPPs.

The European Medicines Agency has launched an investigation into Roche after an inspection found that thousands of potential safety reports, including 15161 deaths, connected to Roche medicines had not been evaluated to determine whether they should be reported to regulators as adverse drug reactions.

FDA Updates List of Warning Letters

The European Medicines Agency's Committee for Orphan Medicinal Products made recommendations for nine orphan drug designations during its June 2012 meeting. Included in COMP's recommendations were four designation applications for rare forms of lipodystrophy.

European and US associations call for continued vigilance against the threat of counterfeit medicines.

BIO is calling for a more patient-centric approach to user-fee reauthorization.

Implementing quality by design makes the determination of quality metrics across CMOs and sponsors essential.
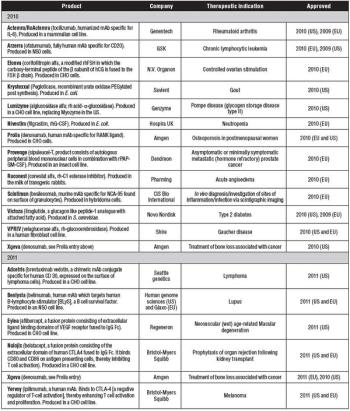
A review of new biologic drug approvals over the years, featuring highlights from 2010 and 2011.

Leading industry collaborators outline top 10 best practices for human error reduction.

In a special anniversary interview, Washington Editor Jill Wechsler speaks with with FDA Deputy Commissioner Deborah Autor about where the agency is headed.

The contract provider needs to know as much as the NDA holder.

A review of key industry shifts and promises for the future.
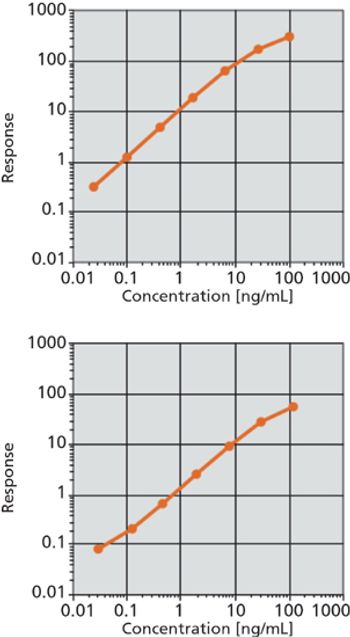
The authors discuss a new, rapid immunoassay for the detection of biomarkers.

Formulators and developers are at the heart of the industry's basic premise-they are saving lives.

Industry experts discuss significant achievements. Plus: What's in store for the future.

The European Federation of Pharmaceutical Industries and Associations (EFPIA) has welcomed the launch of a EUR 223.7-million ($276.5 million) program to tackle antimicrobial resistance and speed up the development of new antibiotics.

ICH Q11, the anticipated guideline from the International Conference on Harmonization, titled Development and Manufacture of Drug Substances, has achieved international consensus. Q11 has been one of the fastest guidelines to move through the ICH harmonization process.

The European Federation of Pharmaceutical Industries and Associations (EFPIA) has formally adopted a memorandum of understanding (MoU) with key partners for a harmonised, European system for medicines verification.