
Prefilled-syringe line features automation and novel disinfection techniques.

Prefilled-syringe line features automation and novel disinfection techniques.

Reduced carryover risk and close attention to particulate control result in greater patient safety through the use of single-use systems.
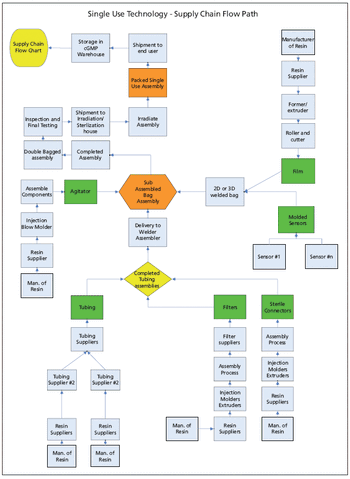
The authors suggest techniques for mitigating risk and securing the supply chain for single-use components used in biopharmaceutical manufacturing.

Have FDA initiatives improved manufacturing quality?

Latin America's diverse growing market seeks regulatory harmonization.

Opioid abuse generates calls for efforts to curb distribution.

EU authorities are stepping up their efforts to incorporate QbD principles.

The authors describe the growth characteristics of human mesenchymal stem cells cultured in a stirred-tank bioreactor.

The New Jersey-licensed specialty pharmacy recalls magnesium sulfate products and halts all production operations.

INTERPOL and 29 of the world's largest pharmaceutical companies have joined forces in an initiative to battle counterfeit drugs.
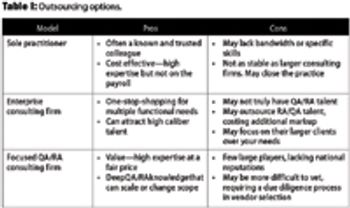
This article examines the options to best match needs and spending for quality and regulatory leadership.

The authors present solutions based on a review of current service offerings and their audit experience.

As biopharmaceutical/pharmaceutical companies increase their development of biologic-based drugs, companies providing analytical instrumentation and laboratory testing goods and services are, in turn, offering improved tools for biologic characterization, biomanufacturing, and related testing.
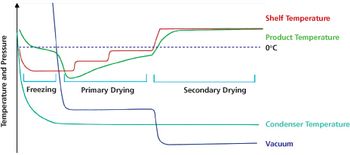
Optimized freeze-drying cycles can offer scientific and business advantages.

Vaccine development is benefiting from manufacturing advances.

Aggregate formation is influenced by multiple aspects of the bioproduction process but can be mitigated by good process design and control.

BioPharm International spoke with industry experts about the effect FDA's 2011 process validation guidance has had on industry.

USP's focus in 2013 involves standards relating to organic impurities, measurement of residual DNA and host-cell proteins in biotechnology products, and elemental impurities.
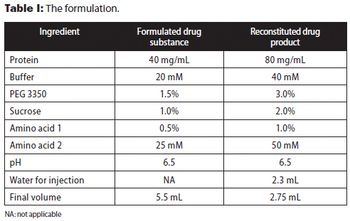
The authors present approaches used to reduce reconstitution time of a lyophilized high-concentration protein drug product.

A QbD paradigm advances process understanding in development and manufacturing.

Applying quality-by-design and process analytical technology facilitates process understanding and control of various operations in lyophilization.

Discussions are underway as the pharmaceutical sector calls for greater consistency in the global monitoring of GMP compliance and quality testing of APIs and finished medicines.

The EMA's Committee for Medicinal Products for Human Use (CHMP) has recommended Sanofi's six-in-one pediatric vaccine for marketing authorization.

The European Commission claims that Johnson & Johnson and Novartis may have breached European antitrust rules.

Shortages spur efforts to overhaul manufacturing oversight.