
Additional approvals in December have helped to outpace a recent high set in 2011.

Additional approvals in December have helped to outpace a recent high set in 2011.

FDA talks about the changing scope of regulatory science.

Therapeutics targeting epigenetic mechanisms of disease will change the pharmaceutical marketplace.

Shortages spur efforts to overhaul manufacturing oversight.

The EU fine-tunes the Falsified Medicines Directive.

What the industry's future holds and what needs to be done to get there.

Neil Lewis, chief technology officer at Malvern Instruments, talks about the challenges associated with biologics.

A recent ISPE guidance provides a baseline for the design of quality laboratory facilities.

The management board of the European Medicines Agency (EMA) has endorsed the agency?s work programme and budget for 2013, which includes a budget of EUR231.6 million, a slight increase over 2012.

Mylan Inc. has issued a recall of three lots of the painkiller tablets, hydrocodone bitartrate and acetaminophen, USP 10 mg/500 mg, due to the potential risk that these lots (lots 3037841, 3040859 and 3042573) may exceed the potency stated on the label.

FDA has issued a draft guidance to assist applicants in the submission of summary-level clinical-site data.

The Court of Justice of the European Union has dismissed an appeal by AstraZeneca concerning a 2005 decision that found the company guilty of abusing its dominant position in the marketplace.

Myriad Genetics reports that the US Supreme Court has granted certiorari agreeing to hear the company's gene patentability case.

A UC Berkeley survey provides insight into biopharma's risk concerns and strategies.

White House and Congress likely to struggle over funding for bio/pharmaceutical regulation.

An introduction to a new series on manufacturing within global markets.
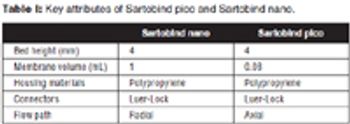
The authors describe the development of an ultra scale-down anion exchange membrane adsorber, and demonstrate scalability to larger-scale devices.

This month, Sharon Strause, an industry consultant, provides a look back at "Computer System Validation Part I: Testing and Verification of Applications Software" by Leonard J. Goren.

The European Commission (EC) is seeking to introduce a black symbol to identify medicines that are subject to additional monitoring.

An operation spanning 16 African countries and conducted by the World Customs Organization (WCO) in partnership with the Institute of Research against Counterfeit Medicines (IRACM) led to the seizure of more than 82 million doses of counterfeit medicines.

USP revises labeling requirements for Heparin.
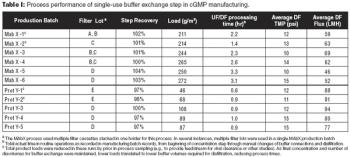
This case study describes the process used to transition from a multi-use system to single-use tangential flow filtration for performing final buffer exchange steps.
Insights on single-use systems implementation and exploitation in biopharmaceutical manufacturing and processing, based on a QbD approach.

Is process-centered organization in biopharmaceutical manufacturing a stepping stone or a stumbling block?

NIBRT's Jayne Telford provides an overview of biopharmaceutical analytics and their accompanying qualification and validation steps.