
FDA and EMA release a draft joint proposal on the development of new drugs to treat Gaucher disease in children.

FDA and EMA release a draft joint proposal on the development of new drugs to treat Gaucher disease in children.

FDA clarifies stability data recommendations for abbreviated new drug applications.

Draft guidance addresses the use of clinical pharmacology studies to determine biosimilarity of biologics.

FDA releases draft guidance on the development of drugs to treat hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia.

The rising incidence of medicine defects and shortages stemming from sub-standard manufacturing is forcing Europe to give higher prominence to more effective inspections procedures.

New formulations and expanded vaccine production are encouraged.

Industry players offer suggestions for quality metrics as FDA continues to try and solve the problem of drug shortages.

Biopharma companies should not overlook India's growing market.

USP releases new and revised standards for organic impurities in medicines for public comment.

The European Medicines Agency's Annual Report highlights the agency's drug approvals, projects, and initiatives for 2013.

EMA notifies EU healthcare professionals of the falsified cancer drug Herceptin.
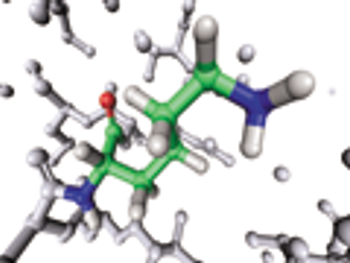
USP evaluates raw materials used in the chemical synthesis of peptides.

New identifiers and tracking requirements aim to block illegitimate products.

As Europe strives to firmly incorporate quality-by-design principles, there are several key issues that still need to be addressed.

Looking to improve patient access to new medicines, EMA creates a pilot project to explore an adaptive licensing approach with real medicines in development.

FDA clarifies recommendations for injectable drug products packaged in vials and ampules.

HHS plan makes progress in ensuring availability of safe vaccines.

Agencies extend successful pilot program to further harmonization of QbD topics.

New guidance from FDA asks for documentation of CMC postapproval manufacturing changes.

Accelerated testing and production create challenges in documenting product quality.

China's regulatory and compliance environment is set to change as the government declares a crackdown on bribery scandals.
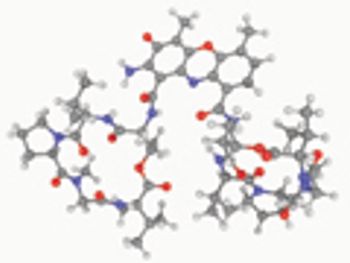
USP evaluates quality attributes for synthetic peptides.
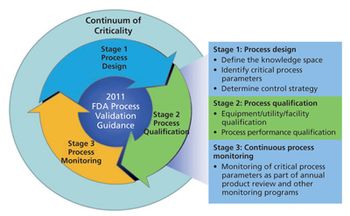
This series presents a practical roadmap in three parts that applies scientific knowledge, risk analysis, experimental data, and process monitoring throughout the three stages of the process validation lifecycle. In Parts I and II, risk analysis and process characterization studies were used to assign criticality risk levels to critical quality attributes and critical process parameters, and the concept of a continuum of criticality was established. In Part III, the author applies the continuum of criticality to develop the process control strategy and move through Stages 2 and 3 of the new process validation lifecycle.
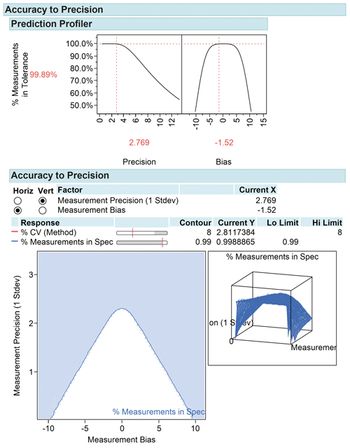
Design of experiment is a powerful development tool for method characterization and method validation.

Manufacturers are taking measures to comply with new package safety rules.