
It is essential to build and maintain a good working relationship between the client and contract research organization.

It is essential to build and maintain a good working relationship between the client and contract research organization.

The current competitive environment is forcing service providers to evaluate their business models and focus on value and performance.
A good understanding of European regulations governing batch release testing will facilitate your collaboration with a contract laboratory.
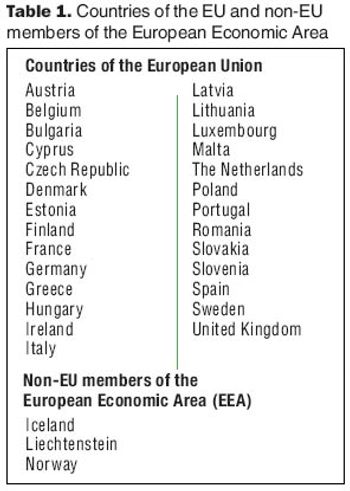
How to successfully balance patient safety with supply-chain management
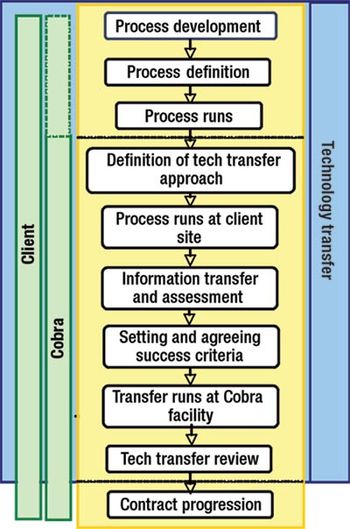
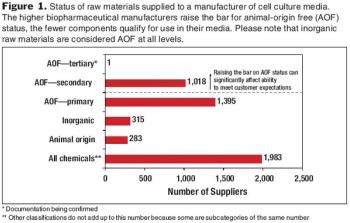
Effective tech transfer can save time and effort in later manufacturing processes.

Outsourcing introduces considerable complexity into ensuring compliance with good manufacturing practices. This article offers FDA guidance for how to ensure compliance in the outsourcing environment.

Communication between contract analytical laboratory and client is extremley important when out-of-specification (OOS) results arise.
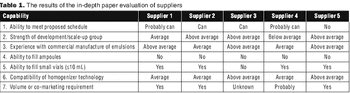
"A systematic approach taken by a company involved making an assessment of internal capabilities, strengths, and needs before the selection process."

What the current lack of venture capital means for the CRO market.

How to ensure smooth technology transfers.
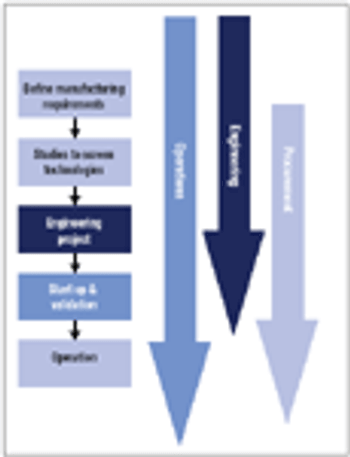
In new disposables projects, it is critical that engineering, procurement, and operations groups work together early on to manage supply chain risk.
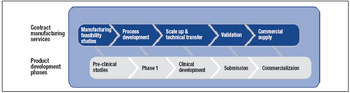
Four reasons why outsourcing may be the best option, and key factors to consider when selecting a provider.

To stave off quality issues associated with production processes, a centralized and enterprise-wide quality management initiative must be enforced.

A closer look at the past year's development in India.

There could be a serious glut of commercial scale mammalian cell culture capacity over the next five years. Then again, there could be a significant shortage. It all depends on how things develop in expression technology, the new product pipeline, and corporate strategies.

If Indian biologics manufacturers can establish a track record for recombinant products, enhance quality image, maintain cost competitiveness, and demonstrate technology transfer and regulatory knowhow, they are likely to be in the middle of the next boom in biologics manufacturing.

When making critical decisions such as whether to build or buy critical capabilities, companies need a decision-making approach that weighs risks and rewards as a science with adequate inputs, repeatable processes, and measurable results. The method must also accommodate the human factor by encouraging wide participation and providing the kind of neutral decision criteria that satisfies participants about the objectivity of the process.
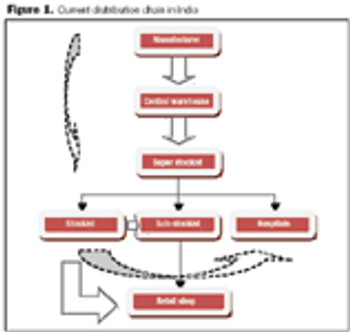
The rapid growth of the pharmaceutical industry in India is yet to create significant changes in the Indian distribution system.

Despite the current regulatory uncertainty, pharmaceutical companies should move forward with planning for serialization and pedigree.

Risk mitigation should be a key aspect of any contract manufacturing organization's business strategy.

What the Indian government is doing to make its biotech sector as strong as its IT sector.

The bioinformatics industry is currently one of the fastest growing fields in India's biotechnology sector. Indian IT companies have several advantages in the bioinformation field and can continue to grow their opportunities worldwide.

It is important to understand critical aspects of the CMO's capabilities. Only by auditing certain key areas can the sponsor be assured of the quality of the materials produced.
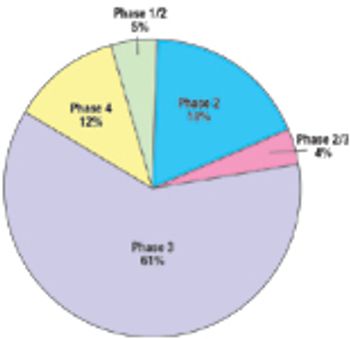
Most local and global clinical research organizations (CROs) consider an operational presence in India as key to their overall business plans. India is clearly on course to become the next hub for clinical trials.
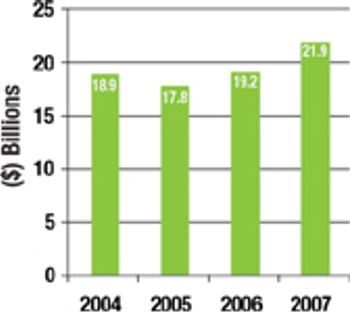
The current overcapacity situation in the bio/pharmaceutical industry is a reminder that CMOs need to come up with business models and value propositions that are based on more than just selling capacity.