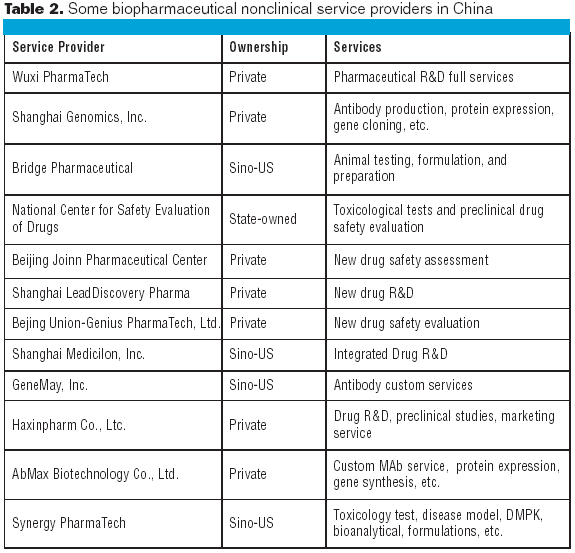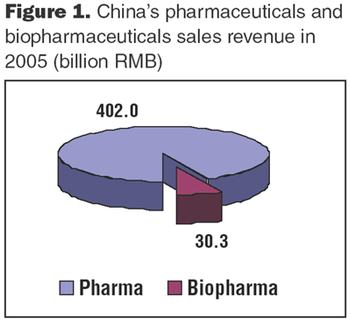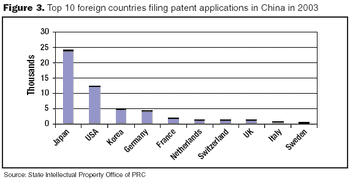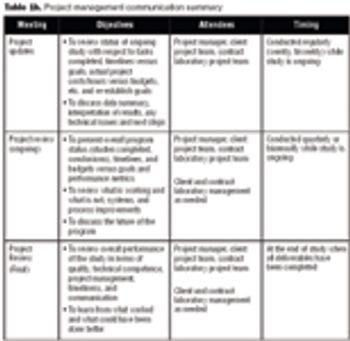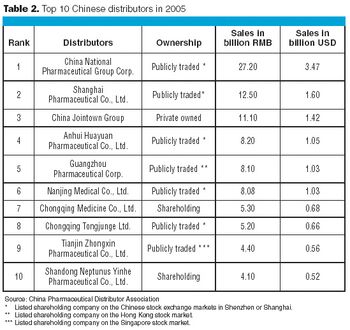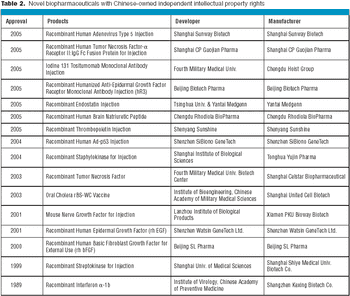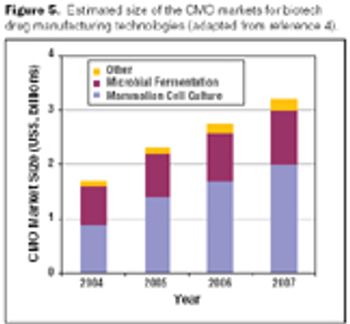
Biologics manufacturing is a technologically complex, highly regulated process. In comparison to small-molecule manufacturing, biologics manufacturing requires far more planning, investment, and skilled personnel and, therefore, can be much riskier. For biotech companies requiring such manufacturing capabilities and experience, partnering with a biologics-focused contract manufacturing organization (CMO) can be a good solution.